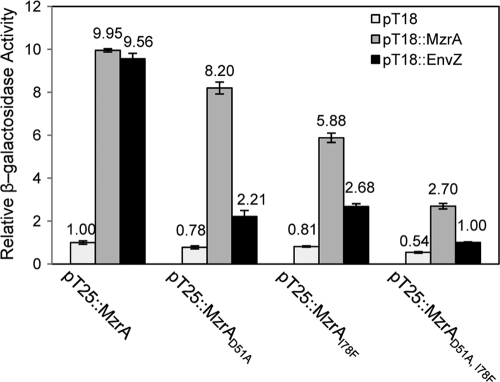
FIG. 7.
In vivo examination of MzrA-MzrA and MzrA-EnvZ interactions. Protein-protein interactions were monitored using a bacterial two-hybrid system involving transcomplementation of T18 and T25 adenylate cyclase fragments from Bordetella pertussis. The two adenylate cyclase fragments, expressed separately from two compatible plasmids, were produced as fusion proteins containing MzrA or EnvZ (13). Interactions between fusion proteins restored the adenylate cyclase activity, allowing the production of chromosomally expressed LacZ in a Δcya background. Thus, measurement of the β-galactosidase activity reflects the extent of MzrA-MzrA and MzrA-EnvZ interactions. Maximum β-galactosidase activity occurs in the presence of T18::MzrA-T25::MzrA (MzrA dimer formation) or T18::EnvZ-T25::MzrA (MzrA-EnvZ heteromer formation) proteins. Single-amino-acid substitutions in the MzrA periplasmic domain interfere with either the MzrA-EnvZ (D51A) interaction or both MzrA-MzrA and MzrA-EnvZ (I78F) interactions.