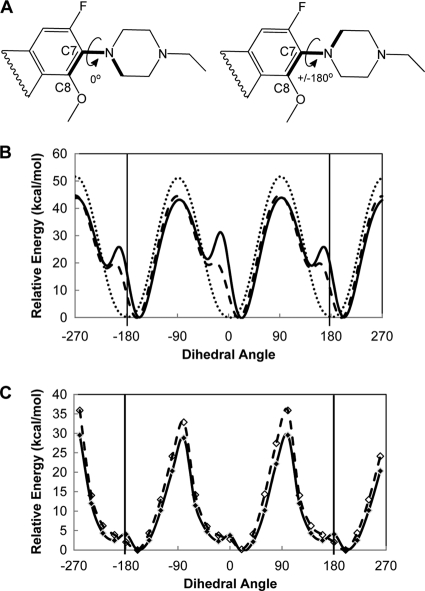
FIG. 5.
Calculated energies for different dihedral angles of the quinolone core and C-7 ring about the C-7-N bond for energy-minimized structures. (A) Two-dimensional representations of partial fluoroquinolone structures showing the three bonds (boldface) used to calculate dihedral angles of rotation for the two ring systems about the C-7-N bond. The structure on the left shows a dihedral angle of 0°. Clockwise rotation of the C-7 ring to +180° (curved arrow) or counterclockwise rotation of the ring to −180° give an equivalent ±180° structure (right structure). (B) Plot of steric energies for 360° rotation of the C-7 piperazine ring about the C-7-N bond from the lowest-energy structure with no incremental energy minimization during rotation for PD161144 (solid line), UING4-257 (dashed line), and ciprofloxacin (dotted line). (C) Plot of energies from energy-minimized structures of PD161144 (solid line) and UING4-257 (dashed line) at 20° increments of the dihedral angle about the C-7-N bond. The three bonds forming the dihedral angle about the C-7-N bond (shown in panel A) were locked at 20° increments; energy minimization was then performed to provide the lowest-energy conformation of each structure at each angle, and the total energy was calculated at each angle. The relative energy (y axis) is the difference between the calculated total energy at each angle and the lowest calculated energy (the angle where the lowest-energy structure is found); the energy minimum is thus zero for the lowest-energy structure on the relative scale.