Abstract
The use of microbicides is a promising approach for the prevention of HIV-1 transmission. Unfortunately, various candidates failed in clinical trials. In some cases, the candidate microbicide even resulted in enhanced virus transmission. Therefore, there is an urgent need to develop more predictive preclinical strategies to anticipate the in vivo efficiency/toxicity rate, including in vitro assays that evaluate effects on epithelial integrity and inflammation. The present study aims to identify potential safety issues concerning the use of microbicides and excipients commonly used in vaginal microbicide preparations. The toxicities of various active pharmaceutical ingredients (APIs; TMC-120, UC-781, tenofovir [PMPA], PRO-2000, and glycerol monolaurate [GML]) and excipients (preservatives, cosolvents, surfactants, and cyclodextrins) were evaluated using an in vitro dual-chamber model and uterine cervical explants. Epithelial viability and permeation of fluorescent virus-sized beads, as well as induction of interleukin-8 (IL-8; as a sensitive marker of an inflammatory response), were assessed. Surprisingly, cell viability and epithelial layer integrity were compromised by most excipients at concentrations near the typical concentration used in vaginal gels, and a significant increase in the production of IL-8 was observed at subtoxic concentrations. Within the APIs, TMC-120, UC-781, and PMPA showed higher selectivity indices than PRO-2000 and GML. In conclusion, identification of safety issues concerning the use of pharmaceutical excipients could help to formulate less toxic vaginal microbicide preparations.
There is an urgent need to develop safe, effective, and acceptable vaginal products for the prevention of sexually transmitted infections (STIs), including HIV-1 infections. Since barrier methods, such as male and female condoms, are, unfortunately, not yet sufficiently accepted, efficacious vaccines and topical microbicides are needed (4, 6, 8). The vagina has been explored as a suitable site for delivery of drugs used for the treatment of local, female-specific infections, such as vaginitis, bacterial vaginosis, and candidiasis (16, 39). Vaginally administered formulations are also being developed to provide protection against various STIs, including HIV-1 infections. Unfortunately, in the past few years, several phase IIB/III clinical trials of candidate microbicides (i.e., nonoxynol-9 [N-9], cellulose sulfate [CS], Savvy [C31G], a carrageenan-based vaginal gel [Carraguard], and a vaginal gel containing a polyanionic entry inhibitor [PRO-2000]) resulted in rather disappointing findings, showing either no significant reduction or even an increase in HIV-1 transmission (10, 20, 29, 32, 35).
Various classes of antiretroviral compounds (ARVs) with activities against HIV are available, and new drugs are still being developed. Their chemical diversity requires the use of different and often compound-specific formulation strategies to make them suitable for use as effective microbicides. Delivery strategies must (i) ensure delivery of an effective concentration, (ii) maintain the active form in the female genital tract, and (iii) be nontoxic and encompass gels, tablets, suppositories, films, and rings (17). Excipients are inactive ingredients needed for efficient in vivo delivery of the active pharmaceutical ingredient (APIs) (16). They ensure specific properties of the formulation, including retention and spreading (e.g., viscosity enhancers), stability (e.g., preservatives and humectants), and solubilizing capacity (e.g., cosolvents, surfactants, and cyclodextrins).
Like any other pharmaceutical drug, candidate microbicides are subject to several stages of efficacy and safety testing before proceeding to phase III clinical trials. Preclinical safety testing includes the assessment of toxicity in cell- and tissue-based assays (including assays with epithelial cell lines, peripheral blood mononuclear cells, primary epithelial cells, and cervical explants). Besides these in vitro and ex vivo models, models involving small animals (mice and rabbits) and nonhuman primates are currently used for safety screening of microbicide candidates. However, there are various ethical and practical issues, such as the increasing number of candidate microbicides and formulation options, the suboptimal reproducibility of the vaginal irritation model, the limited availability of nonhuman primates, and the differences between the animal and human physiology.
In order to reduce the failure rate of microbicide formulations in animal and clinical testing, better in vitro or ex vivo screening tools to identify potential safety issues are needed. Obviously, both the APIs and pharmaceutical excipients used in the formulations may have detrimental effects on the cervicovaginal mucosa, from causing a local inflammation (with influx of potential HIV target cells) to impairing the epithelial barriers, potentially leading to an increased risk of HIV acquisition. While the toxicity profiles of microbicide candidate APIs are already being assessed in in vitro screening assays (cell-based assays, tissue explants), this is not the case for excipients. Selection of excipients is mainly based on their functionality and, if available, data on previous use in vaginal formulations, as can be found in the FDA list of inactive ingredients (9). The latter might be a good reference to use to judge the safety of vaginal preparations intended for short-term use (such as products for the treatment of local infections) but may be less well adapted for the evaluation of products intended for long-term repeated use (such as contraceptives, hygiene products, and microbicides). Currently, safety issues due to excipients may be suspected only during animal testing of complete formulations. Therefore, in vitro safety screening of excipients in early development would be of great assistance to scientists in composing appropriate microbicide formulations.
In the present study, a recently described in vitro dual-chamber model (15) was applied as a screening tool to detect potential safety issues for candidate microbicides and pharmaceutical excipients. Their effects on the viability of a variety of genital epithelial cell lines as well as cervical tissue explants were assessed. In addition, the integrity of the epithelial layer as well as induction of interleukin-8 (IL-8) production by epithelial cells was evaluated. Various candidate microbicides (TMC-120, UC-781, tenofovir [PMPA], PRO-2000, and glycerol monolaurate [GML]) and various classes of excipients (preservatives, cosolvents, surfactants, and cyclodextrins) were evaluated in these assays.
MATERIALS AND METHODS
Epithelial cells.
The cell lines HEC-1A, SiHa, and CaSki were obtained from the American Type Culture Collection (ATCC-LGC Promochem, Teddington, United Kingdom). The uterine HEC-1A cell line originates from a human endometrial adenocarcinoma (23). SiHa cells originate from a uterine cervical carcinoma and have been reported to contain one to two copies of an integrated human papillomavirus type 16 (HPV-16) genome per cell (2). CasKi is a uterine cervical carcinoma cell line containing an integrated HPV-16 genome at about 600 copies per cell, as well as sequences related to HPV-18 (1).
HEC-1A cells were cultured in McCoy's 5A modified medium (Invitrogen, Merelbeke, Belgium) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FBS), further referred to as McCoy's complete medium. SiHa and CaSki cells were cultured in Dulbecco modified Eagle medium (DMEM)-F-12 medium (Invitrogen) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% FBS and is further referred to as complete medium.
Cervical tissue explants.
Cervical tissue was obtained from premenopausal women undergoing planned therapeutic hysterectomy at Ghent University Hospital (written consent was obtained from all tissue donors, according to the local research ethics committee). Cervical explants comprising both epithelial and stromal tissues (diameter, ∼3 mm) were cultured in 96-well flat-bottomed tissue culture plates in RPMI with 10% FBS (19).
Transwell dual-chamber system.
HTS Transwell-96 permeable supports (Corning Costar Corp., Cambridge, MA) developed for high-throughput screening applications were used. The apical chamber of a dual-chamber Transwell system (Fig. 1) with a growth area of 0.143 cm2 (pore size, 3.0 μm) was coated with 200 ng/ml of laminin (Sigma-Aldrich, St. Louis, MO) and air dried. One hundred thousand HEC-1A cells (in 100 μl) were cultured in the apical chamber. The basal chamber contained 150 μl of McCoy's medium. The cells were cultured for 3 days, and the medium in the basal chamber was refreshed on day 2 of the culture.
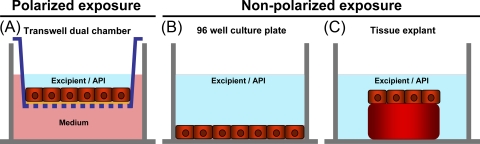
FIG. 1.
Schematic representation of the in vitro models used to evaluate the toxicities of various microbicide candidates and excipients. (A) Nonpolarized exposure setup. Epithelial cells (HEC-1A, CaSki, or SiHa cells) were cultured in 96-well flat-bottomed culture plates. (B) Polarized exposure setup. Assays were performed in a dual-chamber model that consists of an apical chamber (Transwell) and a basal chamber. On top of the permeable membrane, HEC-1A cells were cultured and formed a confluent epithelial layer. In this setup, only the apical side of the HEC-1A layer was exposed to the APIs/excipients. (C) Cervical tissue explant. Cervical explants were cultured in 96-well flat-bottomed culture plates and exposed to the APIs/excipients in a nonpolarized fashion.
APIs.
The nonnucleoside reverse transcriptase inhibitor (NNRTI) UC-781 was kindly provided by A. Van Aerschot, Rega Institute, Leuven, Belgium, whereas the NNRTI TMC-120 (dapivirine) was kindly donated by Tibotec BVBA, Mechelen, Belgium. Stock solutions of these hydrophobic compounds were prepared by dissolution in dimethyl sulfoxide (DMSO) at a concentration of 100 mM. To avoid DMSO-induced toxicity, stock solutions were diluted at least 1,000-fold in culture medium before use. Stock solutions of the nucleotide reverse transcriptase inhibitor (NRTI) PMPA (tenofovir; kindly provided by J. Balzarini, Rega Institute, Leuven, Belgium), GML (monomuls 90-L 12; donated by Cognis Corporation Care Chemicals, Hoofddorp, Netherlands), PRO-2000 (kindly provided by A. Profy, Indevus Pharmaceuticals, Inc., Lexington, MA), and N-9 (Tergitol; Sigma-Aldrich, Bornem, Belgium) were prepared in phosphate-buffered saline (PBS; BioWhittaker, Verviers, Belgium).
Pharmaceutical excipients.
Excipients belonging to various classes were used (Table 1), including five preservatives, consisting of methylparaben, propylparaben, and sorbic acid (Sigma-Aldrich, St. Louis, MO), benzyl alcohol (Acros Organics, Geel, Belgium), and benzalkonium chloride (Certa, Braine-L'Alleud, Belgium); five cosolvents, consisting of ethanol, glycerin, polyethylene glycol 1000 (PEG 1000; Sigma-Aldrich, St. Louis, MO), polyethylene glycol 400 (PEG 400; Acros Organics, Geel, Belgium), and propylene glycol (PG; Certa, Braine-L'Alleud, Belgium); four surfactants, consisting of Cremophor EL (Sigma-Aldrich, St. Louis, MO), d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS 1000; Eastman Chemical Company, Kingsport, TN), polysorbate 80 (Tween 80; Certa, Braine-L'Alleud, Belgium), and sodium lauryl sulfate (SLS; Merck, Darmstadt, Germany); and seven cyclodextrins, consisting of α-cyclodextrin (α-CD), hydroxypropyl-β-cyclodextrin (HP-β-CD), hydroxypropyl-γ-cyclodextrin (HP-γ-CD), and dimethyl-β-cyclodextrin (DM-β-CD) (Sigma-Aldrich, St. Louis, MO), β-cyclodextrin (β-CD; Roquette, Lestrem, France), γ-cyclodextrin hydrate (γ-CD; Acros Organics, Geel, Belgium), and sulfobutyl ether-β-cyclodextrin (SBE-β-CD; Captisol; CyDex Pharmaceuticals Inc., Lenexa, KS). Serial dilutions of these excipients were prepared in complete medium.
TABLE 1.
Excipients belonging to different classes evaluated in the present study
| Product | Class | TC (% [wt/wt])a | Useb |
|---|---|---|---|
| Methylparaben | Preservative | 0.18 | Parenteral, enteral, otic, ophthalmic, rectal, nasal, vaginal |
| Propylparaben | Preservative | 0.02 | Parenteral, enteral, otic, ophthalmic, rectal, nasal, vaginal |
| Sorbic acid | Preservative | 0.05-0.2 | Enteral, dermal, vaginal |
| Benzyl alcohol | Preservative | 2 | Parenteral, enteral, otic, nasal, ophthalmic, vaginal, dermal |
| Benzalkonium chloride | Preservative | 0.01-0.02 | Parenteral, nasal, otic, ophthalmic, vaginal |
| Ethanol | Cosolvent | 1-10 | Parenteral, enteral, ophthalmic, dermal, vaginal |
| Glycerin | Cosolvent/humectant | 1-20 | Parenteral, enteral, otic, dermal, nasal, ophthalmic, vaginal |
| Polyethylene glycol 400 | Cosolvent | 20 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| Polyethylene glycol 1000 | Cosolvent | 20 | Enteral, dermal, vaginal |
| Propylene glycol | Cosolvent | 1-40 | Parenteral, enteral, otic, nasal, ophthalmic, dermal, rectal, vaginal |
| Cremophor EL | Surfactant | 0.1-2 | Parenteral, enteral, ophthalmic, dermal, vaginal |
| TPGS 1000 | Surfactant | 0.1-2 | Enteral, ophthalmic, dermal |
| Polysorbate 80 (Tween 80) | Surfactant | 0.1-2 | Parenteral, enteral, otic, nasal, ophthalmic, rectal, dermal, vaginal |
| Sodium lauryl sulfate | Surfactant | 0.1-2 | Enteral, dermal, vaginal |
| Hydroxypropyl-β-cyclodextrin | Cyclodextrin | 2.5-20 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| α-Cyclodextrin | Cyclodextrin | 0.5-10 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| β-Cyclodextrin | Cyclodextrin | 0.5-10 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| γ-Cyclodextrin hydrate | Cyclodextrin | 0.5-10 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| Hydroxypropyl-γ-cyclodextrin | Cyclodextrin | 0.5-40 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| Dimethyl-β-cyclodextrin | Cyclodextrin | 0.5-40 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
| Sulfobutylether-β-cyclodextrin | Cyclodextrin | 0.5-40 | Parenteral, enteral, ophthalmic, dermal, vaginal, nasal |
The typical concentration used in pharmaceutical formulations.
The type of preparation in which the excipient is used elsewhere (9).
Evaluation of toxicity.
The tissue/cell viability upon exposure to the various APIs and excipients was assessed in three different setups: in nonpolarized and polarized exposures of epithelial cell lines and a nonpolarized exposure in cervical tissue explants. Cytotoxicity was assessed using an enhanced colorimetric cell proliferation/viability assay, performed with a water-soluble tetrazolium-1 (WST-1) cell proliferation kit, according to the manufacturer's instructions (Roche, Vilvoorde, Belgium). The assay is based on the cleavage of the tetrazolium salt WST-1 ({4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate}) to a formazan dye by a complex cellular mechanism. This bioreduction is largely dependent on the glycolytic production of NAD(P)H in viable cells. Therefore, the amount of formazan dye formed directly correlates to the number of viable cells in the culture and can be quantified by measuring the absorbance at 450 nm in a multiwell plate reader. The greater the number of viable, cells, the greater the amount of formazan dye produced following the addition of WST-1.
Nonpolarized exposure to APIs/excipients.
Twenty-five thousand HEC-1A, CaSki, or SiHa cells were seeded in 96-well plates and cultured for 3 days. Afterwards, a serial dilution of an API/excipient was added to the epithelial cells and the mixture was incubated for 24 h. After this exposure, the cells were washed and WST-1 was added. Cell viability was quantified 1 h later by analysis on a microplate reader (Bio-Rad, Tokyo, Japan) at 450 nm. The calculated percentage of viable cells compared to the number of untreated control cells was plotted against the compound concentration, and linear regression analysis was performed using Prism (version 5.02) software for Windows (GraphPad Software, San Diego, CA) to calculate the 50% cytotoxic concentration (CC50).
Polarized exposure to APIs/excipients.
One hundred thousand HEC-1A cells were grown to confluence on HTS Transwell permeable supports. On day 3 of the culture, the apical side of the epithelial layer was exposed to a serial dilution of one of the various APIs or excipients in complete medium for 24 h. The basal chamber contained McCoy's complete medium during the 24-h exposure. Subsequently, the API/excipient was removed, washed once, and replaced by McCoy's complete medium and the WST-1 substrate was added to the basal chamber for 2 h. The viability of the epithelial layer was assessed with the WST-1 assay, as described above.
Cervical tissue explant.
Fresh cervical tissue was obtained after hysterectomy at Ghent University Hospital. Explants were placed in 96-well flat-bottomed tissue culture plates and treated with a serial dilution of an API/excipient for 24 h. Subsequently, the explants were washed once with culture medium, and WST-1 substrate was added for 2 h. Tissue viability was assessed as described above.
Permeability to fluorescent microspheres.
One hundred thousand HEC-1A cells were grown to confluence on HTS Transwell permeable supports. On day 3 of culture, the epithelial layer was exposed to a serial dilution of one of the various APIs or excipients for 24 h. As a 100% toxic control, HEC-1A cells were treated with 1% N-9. Subsequently, the excipient was removed, the cells were washed once, and 100 μl of a 1/20 dilution of fluorescent microspheres (FluoSpheres sulfate microspheres; diameter, 0.1 μm; yellow-green fluorescence [505/515 nm]; 2% solids; Molecular Probes Europe NV, Leiden, Netherlands) was added on top of the epithelial layer in the apical chamber, and the mixture was incubated for another 24 h (37°C, 5% CO2). These microspheres have dimensions similar to those of HIV-1 particles (i.e., a diameter of 100 nm). Afterwards, the medium in the basal chamber was harvested; the presence of fluorescence was assessed with a fluorometer (TriStar LB 941; Berthold Technologies GmbH, Germany) and analyzed with MicroWin 2000 software. The percentage of beads that crossed the treated epithelial layer compared to the number for the 1% N-9-treated control was plotted against the compound concentration, and linear regression analysis was performed using Prism (version 5.02) software for Windows (GraphPad Software) to calculate the 50% toxic concentration (integrity) (CC50, integrity).
Chemokine assay.
One hundred thousand HEC-1A cells were cultured to confluence on Transwell inserts (pore size, 3 μm) for 3 days. Subsequently, the apical side of the epithelial layer was exposed to a serial dilution of one of the APIs or excipients in duplicate wells for 24 h. Culture supernatants were collected from the basal compartment of the dual-chamber system at the end of the 24-h treatment period. The levels of IL-8 (CXCL8) in the culture supernatants were quantified by a traditional colorimetric enzyme-linked immunosorbent assay (ELISA; RayBiotech IL-8 ELISA kit; Tebu Bio, Le Perray en Yvelines, France).
Data processing and statistical analysis.
Data were analyzed to produce arithmetic or geometric means with standard deviations (SDs) using Microsoft Excel or Prism (GraphPad Software) software. Linear regression analysis was performed using GraphPad Prism software to calculate the 50% toxic concentration in all assays. Analysis of variance (ANOVA) and the Student t test were performed to determine the significance of the difference between sets of data. Correlations between the different assays were calculated using the Spearman rank correlation test.
RESULTS
In vitro systems to model the female genital mucosa.
To evaluate the effects of APIs and excipients on the female genital mucosa, various in vitro models were used (Fig. 1). In the first model, a nonpolarized exposure setup, cells of three different cell lines, i.e., HEC-1A (endometrium), CaSki, and SiHa (uterine cervix), were grown in 96-well culture plates and exposed for 24 h to a serial dilution of one of the excipients. Cell viability was assessed using the WST-1 assay. In the second model, a polarized exposure setup, a confluent layer of HEC-1A cells, impermeable for fluorescent beads (diameter, 100 nm, which is the size of an HIV-1 virion), was grown on HTS Transwell inserts (15) and subsequently exposed to a serial dilution of each excipient or API applied on the apical side only for 24 h. This in vitro setup mimics the in vivo application of a microbicide, where the luminal side of the vaginal and uterine cervical epithelium is exposed to the microbicide. Following this exposure and washing of the compound, the viability of the cell layer was assessed using the WST-1 assay and the epithelial integrity by measuring the leakage of fluorescent microspheres into the basal compartment. Finally, the third model consisted of fresh uterine cervical tissue biopsy specimens containing both the epithelium and the underlying stroma in a physiological architecture. Explant tissue was exposed, in a nonpolarized fashion, to a serial dilution of one of the APIs or excipients. This more complex ex vivo model, which is widely used in the microbicide field to evaluate the safety and activity of candidate microbicides, was used as a comparative standard.
Toxicity profiles of candidate microbicides in the various assays.
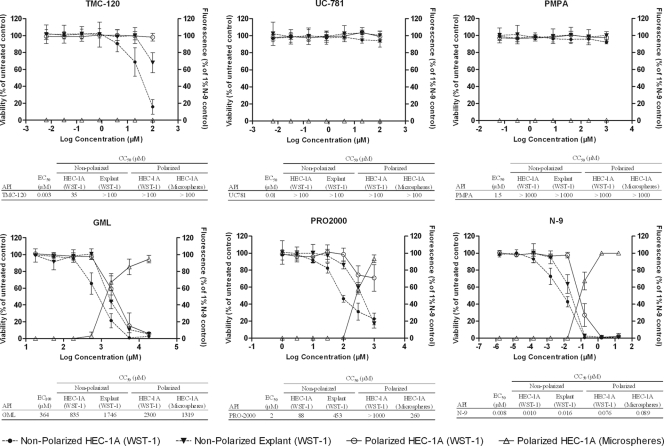
The viability of HEC-1A epithelial cells and cervical tissue was assessed after a nonpolarized exposure to serial dilutions of five candidate microbicides (TMC-120, UC-781, PMPA, PRO-2000, and GML) and a compound that has been clearly associated with an increased risk of HIV acquisition, i.e., N-9. In addition, the viability and integrity of the HEC-1A epithelial layer were assessed following a polarized exposure to the APIs. In Fig. 2, dose-response curves and the resulting half-maximal cytotoxic concentrations (CC50s) are shown. In vitro anti-HIV half-maximal effective concentrations (EC50s) were added to allow interpretation of the toxicity data. The NNRTI TMC-120 has an EC50 of about 0.003 μM in a dendritic cell/T-cell coculture model (34) and had a CC50 of 35 μM (selectivity index [SI], >11,666) in the nonpolarized exposure setup. Remarkably, no cytotoxicity or decreased epithelial layer integrity was observed in the polarized exposure of HEC-1A cell layers to up to 100 μM (SI, >33,000), but some cytotoxicity was evident in the cervical explant model at 100 μM. Higher concentrations were not tested due to solubility issues. The NNRTI UC-781 (EC50, ∼0.01 μM in a dendritic cell/T-cell coculture model) and the NRTI PMPA (EC50, ∼1.5 μM in the same model) (34) displayed no cytotoxicity at the evaluated concentrations (CC50s, >100 μM [SI, >10,000] and >1,000 μM [SI, >666], respectively) in all assays. PRO-2000, a polyanionic entry inhibitor (EC50, ∼2 μM in a dendritic cell/T-cell coculture model) (unpublished data), displayed lower SIs, showing a CC50 of 88 μM (SI, 44) in the nonpolarized exposure setup, whereas in the polarized exposure setup, a reduced viability was observed at 300 μM (CC50, >1,000 μM [SI, >500]). Nevertheless, the epithelial integrity was lost, showing a CC50, integrity of 260 μM (SI, 130), and a CC50 of 458 μM (SI, 229) was observed in the cervical explant model. Next, GML, which has an effective concentration against HIV of ∼364 μM (31), showed a CC50 of 835 μM (SI, <2) in the nonpolarized exposure setup and a CC50 of 2,300 μM (SI, <6) in the polarized HEC-1A cell exposure setup. The epithelial integrity toward microspheres was impaired starting at 547 μM GML, with the CC50, integrity being 1,319 μM (SI, <4). In the cervical explant model, it showed a CC50 of 1,746 μM (SI, <5). Finally, N-9 (EC50s, 0.0032 to 0.0081 μM [40]), which was used as a control, showed a CC50 of 0.01 μM (SI, 1) in the nonpolarized exposure setup, a CC50 of 0.076 μM (SI, 10) in the polarized exposure setup, and a CC50, integrity of 0.089 μM (SI, 11) in the bead permeation assay. In the cervical explant model, N-9 showed a CC50 of 0.016 μM (SI, 2).
FIG. 2.
Toxicity profiles of various candidate microbicides (APIs). HEC-1A cells were cultured in 96-well culture plates (nonpolarized exposure) and HTS Transwell inserts (polarized exposure) and exposed to the indicated microbicides (TMC-120, UC-781, PMPA, GML, PRO-2000, and N-9). Toxicity toward HEC-1A epithelial cells and cervical tissue was assessed using WST-1. A change in epithelial layer integrity was assessed using fluorescent microspheres. The viability of the HEC-1A epithelial layer in the nonpolarized exposure setup (solid circles), the viability of the tissue explants in the nonpolarized exposure setup (solid triangles), and the viability of the HEC-1A epithelial layer in the polarized exposure setup (open circles) compared to the viability of an untreated control (left y axis) are shown. The open triangles show the permeation of beads through the HEC-1A epithelial layer in the polarized exposure setup compared to the results for a 1% N-9-treated control (right y axis). Results are means ± standard deviations from at least 2 independent experiments in which each condition was tested in triplicate.
Preliminary comparison of toxicity profiles of the excipients in various epithelial cell lines and assays.
The safety of a selection of excipients belonging to different classes (i.e., preservatives, cosolvents, surfactants, and cyclodextrins) was evaluated in the nonpolarized cytotoxicity assays, using three different cell lines (HEC-1A, CaSki, and SiHa) and cervical explants. In addition, both cytotoxicity and functional layer integrity were assessed in the polarized setup with HEC-1A cells. Epithelial cell and tissue cytotoxicities were measured using the WST-1 assay, whereas epithelial layer integrity was assessed in a bead permeation assay and compared to that achieved with a 1% N-9-treated control. Results are shown in Table 2.
TABLE 2.
Comparison of CC50s (viability assay) and the concentrations which raise the permeation of fluorescent beads to 50% of that for a 1% nonoxynol-9-treated control (epithelial layer integrity assay) obtained in the various assaysa
| Product | TC (% [wt/wt/]) | CC50 (% [wt/wt])a |
|||||
|---|---|---|---|---|---|---|---|
| Nonpolarized assay (WST-1) |
Polarized assay with HEC-1A cells |
||||||
| SiHa cells | CaSki cells | HEC-1A cells | Explant | WST-1 | Microspheres | ||
| Methylparaben | 0.18 | 0.06 (0.05-0.07) | 0.05 (0.05-0.06) | 0.05 (0.04-0.06) | 0.06 (0.05-0.08) | 0.66 (0.58-0.72) | 0.67 (0.58-0.85) |
| Propylparaben | 0.02 | 0.04 (0.03-0.05) | 0.03 (0.03-0.03) | 0.03 (0.01-0.07) | 0.03 (0.01-0.06) | 0.07 (0.06-0.08) | 0.07 (0.06-0.07) |
| Ethanol | 1-10 | 4.59 (2.20-5.93) | 4.14 (3.73-4.96) | 3.13 (2.42-3.70) | 8.56 (7.62-9.49) | >30 (>30) | >30 (>30) |
| Glycerin | 1-20 | 11.34 (9.45-12.68) | 13.75 (11.69-16.25) | >20 (>20) | 11.17 (10.60-11.54) | 16.46 (10.87-19.74) | 18.41 (17.48-19.49) |
| Polyethylene glycol 400 | 20 | 7.08 (6.22-7.56) | 8.79 (8.12-9.85) | 6.71 (5.32-7.83) | 4.77 (3.17-6.10) | 23.52 (18.00-29.17) | 15.20 (14.07-16.16) |
| Polyethylene glycol 1000 | 20 | 8.75 (7.02-9.68) | 10.35 (6.88-13.40) | 9.30 (7.83-12.41) | 7.87 (7.08-9.42) | 23.99 (18.17-28.77) | 18.01 (17.41-19.12) |
| Cremophor EL | 0.1-2 | <0.5 (<0.5) | <0.5 (<0.5) | <0.5 (<0.5) | 0.83 (0.69-1.01) | 2.19 (0.97-4.51) | 1.92 (1.29-3.67) |
| TPGS 1000 | 0.1-2 | <0.05 (<0.05) | <0.05 (<0.05) | <0.05 (<0.05) | 0.07 (0.04-0.11) | <0.05 (<0.05) | <0.05 (<0.05) |
| Hydroxypropyl-β-cyclodextrin | 2.5-20 | 8.58 (8.33-8.85) | 6.59 (5.71-7.98) | 7.25 (6.22-8.00) | 8.63 (8.26-8.17) | 8.23 (8.02-8.58) | 9.41 (7.85-12.38) |
The geometric means and ranges of three independent experiments performed in triplicate wells are shown. The nonpolarized exposure setup was performed with HEC-1A, CaSki, and SiHa cells. The CC50 obtained in the cervical tissue explant model is the concentration which reduces the tissue viability to 50% compared with that for the untreated control (measured using WST-1). The polarized exposure setup was performed with HEC-1A cells. The last column shows a polarized exposure setup with HEC-1A cells, and the CC50 reflects the concentration which raises the permeation of fluorescent beads to 50% of that for a 1% nonoxynol 9-treated control (100% toxicity).
Comparison of the sensitivities of the various epithelial cell lines (HEC-1A, CaSki, and SiHa) to the toxic effects of the excipients in the nonpolarized setup showed only a few, statistically nonsignificant differences between the various cell lines for all except one excipient (i.e., glycerin), which appeared to be selectively less toxic toward HEC-1A cells (P < 0.05, ANOVA). Furthermore, the CC50 values obtained in the explant model were not significantly (P > 0.05, t test) different from the values obtained in the nonpolarized exposure setup with HEC-1A cells for any of the excipients except glycerin and Cremophor EL. In contrast, the CC50 values obtained in the polarized exposure setup analyzed either with the viability assay or with the bead permeation assay in general showed higher CC50 values compared to those obtained in the nonpolarized exposure setup, with the difference being significant for methylparaben, ethanol, polyethylene glycol 400, and polyethylene glycol 1000.
Toxicity rank order of various excipients.
On the basis of these preliminary results, toxicity testing was continued in three assays, i.e., the two viability assays with the HEC-1A cell line (the nonpolarized and polarized exposure setups) and the epithelial layer integrity assay (the beads permeation assay).
Within each class of excipients, a rank order based on the CC50 values relative to the typical concentration (TC) used for clinical applications can be made (Table 3). Among the preservatives, cell viability and epithelial layer integrity were compromised the most by benzalkonium chloride, benzyl alcohol, and propylparaben but were compromised less by methylparaben and sorbic acid, for which the toxic concentration was higher than the TC, at least in the polarized setups. The tested cosolvents also displayed toxicity at clinically used concentrations, with ethanol and glycerin having better profiles than the polyethylene and propylene glycols. All tested surfactants exerted toxic effects at concentrations equal to or lower than the typical concentration used in formulations: sodium lauryl sulfate = TPGS 1000 > polysorbate 80 > Cremophor EL (low toxicity). Among the cyclodextrins, the following relative rank order for toxicity was observed: dimethyl-β-CD > α-CD, β-CD > γ-CD > hydroxypropyl-β-CD > sulfobutylether-β-CD > hydroxypropyl-γ-CD (in fact, hydroxypropyl-γ-CD was the only one with relatively low toxicity).
TABLE 3.
CC50 values obtained in three assays with HEC-1A cellsa
| Product | Class | TC (% wt/wt) | CC50 obtained in HEC-1A cells (% [wt/wt])a |
||
|---|---|---|---|---|---|
| Nonpolarized assay (WST-1) | Polarized assay |
||||
| WST-1 | Microspheres | ||||
| Methylparaben | Preservative | 0.18 | 0.05 (0.04-0.06) | 0.66 (0.58-0.72) | 0.67 (0.58-0.85) |
| Propylparaben | Preservative | 0.02 | 0.03 (0.01-0.07) | 0.07 (0.06-0.08) | 0.07 (0.06-0.07) |
| Sorbic acid | Preservative | 0.05-0.2 | 0.58 (0.49-0.64) | 1.22 (0.70-2.00) | 0.86 (0.65-1.10) |
| Benzyl alcohol | Preservative | 2 | 0.13 (0.07-0.32) | 0.66 (0.57-0.73) | 0.87 (0.63-1.09) |
| Benzalkonium chloride | Preservative | 0.01-0.02 | <0.001 (<0.001) | 0.003 (0.002-0.003) | <0.001 (<0.001) |
| Ethanol | Cosolvent | 1-10 | 3.13 (2.42-3.70) | >30 (>30) | >30 (>30) |
| Glycerin | Cosolvent/humectant | 1-20 | >20 (>20) | 16.46 (10.87-19.74) | 18.41 (17.48-19.49) |
| Polyethylene glycol 400 | Cosolvent | 20 | 6.71 (5.32-7.83) | 23.52 (18.00-29.17) | 15.20 (14.07-16.16) |
| Polyethylene glycol 1000 | Cosolvent | 20 | 9.30 (7.83-12.41) | 23.99 (18.17-28.77) | 18.01 (17.41-19.12) |
| Propylene glycol | Cosolvent | 1-40 | 11.19 (9.90-11.90) | 16.18 (15.21-17.52) | 23.21 (20.02-26.93) |
| Cremophor EL | Surfactant | 0.1-2 | <0.5 (< 0.5) | 2.19 (0.97-4.51) | 1.92 (1.29-3.67) |
| TPGS 1000 | Surfactant | 0.1-2 | <0.05 (<0.05) | <0.05 (<0.05) | <0.05 (<0.05) |
| Polysorbate 80 | Surfactant | 0.1-2 | 0.04 (0.03-0.07) | 0.38 (0.22-0.58) | 0.92 (0.57-1.29) |
| Sodium lauryl sulfate | Surfactant | 0.1-2 | <0.05 (<0.05) | <0.05 (<0.05) | <0.05 (<0.05) |
| Hydroxypropyl-β-cyclodextrin | Cyclodextrin | 2.5-20 | 7.25 (6.22-8.00) | 8.23 (8.02-8.58) | 9.41 (7.85-12.38) |
| α-Cyclodextrin | Cyclodextrin | 0.5-10 | 1.66 (1.05-2.47) | 1.80 (0.86-2.52) | 2.12 (2.00-2.38) |
| β-Cyclodextrin | Cyclodextrin | 0.5-10 | 1.50 (1.12-2.61) | 2.59 (2.16-2.85) | 2.52 (2.27-2.97) |
| γ-Cyclodextrin hydrate | Cyclodextrin | 0.5-10 | 4.65 (2.37-6.99) | 8.62 (6.57-10.71) | 6.77 (5.19-8.91) |
| Hydroxypropyl-γ-cyclodextrin | Cyclodextrin | 0.5-40 | 15.35 (12.05-21.00) | >40 (>40) | >40 (>40) |
| Dimethyl-β-cyclodextrin | Cyclodextrin | 0.5-40 | 0.15 (0.14-0.16) | 0.19 (0.15-0.23) | 0.21 (0.17-0.29) |
| Sulfobutylether-β-cyclodextrin | Cyclodextrin | 0.5-40 | 10.77 (9.41-11.84) | 14.13 (13.13-14.78) | 17.52 (15.67-20.77) |
The three assays comprised two cytotoxicity assays and one functional assay. The geometric means and ranges of three independent experiments performed in triplicate wells are shown. The CC50 values in the nonpolarized and polarized assays reflect the concentration which reduces the cell viability to 50% compared to that for the untreated control (measured using WST-1). The CC50 value in the microsphere permeation assays reflects the concentration which raises the permeation of fluorescent microspheres to 50% of that for a 1% nonoxynol-9-treated control (100% toxicity).
The CC50 values for viability obtained in the polarized exposure setup were higher than those obtained in the nonpolarized exposure setup for all compounds except glycerin. The difference between the two assays was statistically significant for methylparaben, benzyl alcohol, ethanol, the polyethylene and propylene glycols, hydroxypropyl-γ-CD, and sulfobutylether-β-CD (P < 0.05, t test). The CC50 values obtained in the polarized WST-1 assay did not differ significantly from those obtained in the microsphere permeation assay (CC50, integrity).
The Spearman rank correlation test was used to investigate the possible correlations between the toxicity rankings in the various assays. The excipient ranking of the polarized exposure assay was significantly correlated with the rankings of the nonpolarized assay (correlation coefficient, 0.90; P < 0.001), of the bead permeation assay (correlation coefficient, 0.97; P < 0.001), and with the tissue explant model (correlation coefficient, 0.70; P = 0.043). The excipient ranking of the nonpolarized exposure assay was also correlated with the rankings of the bead permeation assay (correlation coefficient, 0.91; P < 0.001) and with the tissue explant model (correlation coefficient, 0.91; P = 0.001).
Production of interleukin-8.
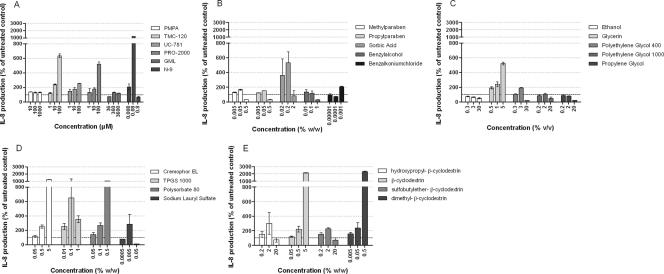
IL-8, a proinflammatory chemokine, was quantified by ELISA in culture supernatants collected from the basal chamber of the dual-chamber system containing a confluent HEC-1A epithelial layer that was treated with either the APIs (Fig. 3A) or the various classes of excipients (Fig. 3B to D). The treatment was performed in a polarized way (apical side) for 24 h and at low concentrations, since we previously showed that IL-8 production is a more sensitive marker of toxicity than either viability or permeability. Treatment with N-9, known to induce a proinflammatory response (11, 12), showed a strong increase in the level of IL-8 production starting at 0.008 μM (i.e., 10 times lower than the CC50 in the polarized WST-1 assay). Higher concentrations of N-9 induced a decrease in the level of IL-8 secretion, most probably as a result of cell death. Treatments with UC-781 and TMC-120 showed small increases in the level of IL-8 production starting at 10 μM. None of the APIs showed cytotoxicity at 100 μM in the polarized WST-1 assay, and only TMC-120 had a CC50 of 35 μM in the nonpolarized WST-1 assay. PRO-2000 increased the level of IL-8 production starting at 10 μM (10 times below that obtained in the nonpolarized WST-1 assay and >100 times below the polarized WST-1 assay CC50). Rather remarkably, GML did not affect IL-8 production even at a cytotoxic concentration. Among the preservatives, sorbic acid and benzalkonium chloride treatments produced strong increases in IL-8 secretion at 0.2% and 0.001%, respectively; but methylparaben, propylparaben, and benzyl alcohol were inactive in this regard and increased IL-8 secretion at up to 0.5 and 1%. Of the cosolvents, only glycerin and PEG 400 increased the level of IL-8 secretion at 5% and 3%, respectively. All surfactants induced a strong increase in IL-8 production (Cremophor EL at 0.5%, polysorbate 80 at 0.5%, TPGS 1000 at 0.01%, and SLS at 0.005%). Finally, dimethyl-β-CD and β-CD increased IL-8 production at 0.5% and 5%, respectively (Fig. 3).
FIG. 3.
Patterns of IL-8 release into basal chamber culture supernatants of HEC-1A epithelial cells cultured in Transwell inserts and treated for 24 h with various doses of APIs or excipients. (A) APIs; (B) preservatives; (C) cosolvents; (D) surfactants; (E) cyclodextrins. IL-8 secretion into culture basal medium is expressed as a percentage of the level of secretion for an untreated control (100%). The mean baseline level of IL-8 production of the untreated control wells was 38 pg/ml (95% confidence interval, between 31 and 46 pg/ml). Mean relative levels of secretion and standard deviations are shown. The experiment was performed in duplicate wells.
Clearly, for some APIs (e.g., UC-781 and PRO-2000) and some excipients (e.g., sorbic acid, glycerin, Cremophor EL, polysorbate 80, and cyclodextrins), the IL-8 assay unveils a presumed proinflammatory effect at concentrations that induce no or limited cytotoxicity in viability or permeation assays. Conversely, other compounds (e.g., GML, the parabens, and the polyethylene glycols) fail to induce IL-8 secretion at any concentration used, whereas viability and permeation assays indicate clear toxic effects over a similar concentration range.
DISCUSSION
The vaginal mucosa is commonly exposed to contraceptives, feminine-care products, and drugs for the treatment of female-specific conditions (16). Hence, irritation of epithelial surfaces due to chemical insult remains a concern. For the prevention of HIV infection, a safe and efficacious anti-HIV microbicide is highly desirable but is not yet available. Recently, four phase IIB/III trials of candidate microbicides (i.e., cellulose sulfate, Savvy, Carraguard, and PRO-2000) showed no reduction or even an increased risk of acquiring HIV (10, 20, 29, 32). These disappointing findings suggest that there is a need for more appropriate in vitro assays to predict in vivo safety issues.
Several in vitro and ex vivo models are currently being explored (5, 11, 13, 15, 19, 22, 27, 34). In vitro assays have the advantage that they are quick and sensitive and can be used for high-throughput screening in early discovery and development. Unfortunately, until now, it has been very difficult to state which in vitro assay best predicts in vivo toxicity. The microbicides that failed in phase IIB/III trials were considered safe in preliminary short-term phase I safety trials and had shown activity against HIV-1 in vitro and sometimes even in macaque models (24, 26, 36, 37). Nevertheless, they resulted in either no benefit or possibly increased HIV-1 infection during the phase IIB/III clinical trials. In the case of the CS phase I safety trial, the gel was found to be safe for vaginal use (36). However, the subsequent phase III trial was closed prematurely due to a nonsignificantly higher incidence of HIV infections in the treated arm than in the placebo arm (20). To date, it is unclear whether or not the failure of CS in the clinical trial was a result of the lack of activity in vivo or enhancement of HIV transmission. Subsequent in vitro research revealed that CS disrupts epithelial tight junctions, resulting in the enhancement of HIV transmission at concentrations lower than those used in the clinical trial (27). This raises the question whether the evaluations performed in phase I clinical trials are sensitive enough to predict the safety outcomes for microbicides.
Here, we report the successful elaboration of an in vitro dual-chamber Transwell model as a tool for rapid screening for epithelial toxicity. In this model, epithelial cell viability was assessed using a colorimetric assay (the WST-1 assay). Epithelial layer integrity was determined by measuring the diffusion of 0.1-μm-diameter sulfate microspheres (FluoSpheres), providing a useful noninfectious alternative as a means to assess epithelial barrier function toward HIV (15). Various microbicide candidates (N-9, TMC-120, UC-781, PMPA, PRO-2000, and GML) and excipients were tested in these assays, and toxicity profiles were generated.
As expected, nonoxynol-9 caused epithelial layer disruption and induced inflammation at doses used in clinical trials (3, 12, 21, 35); the present data are also consistent with previous in vitro results (13). The reverse transcriptase inhibitors (RTIs) PMPA, UC-781, and TMC-120 showed seemingly high selectivity indices. These observations are in line with those described in previous reports showing similarly high selectivity indices for PMPA, TMC-120, and UC-781 in other simple in vitro models of cell toxicity (13, 34, 38). Nevertheless, at higher (μM) concentrations, both TMC-120 and UC-781 induced some IL-8 production. Moreover, at present it is not yet clear what the in vivo active concentrations of these RTIs will be when they are used as microbicides, and hence, their real selectivity indices remain unknown. Furthermore, the polyanionic entry inhibitor PRO-2000 had a less promising safety profile. It interfered with epithelial layer integrity and induced IL-8 secretion at concentrations that are only about 10-fold higher than the in vitro EC50s. In contrast to the present results for PRO-2000, Weber et al. have shown a high selectivity index for PRO-2000, with an EC50 of 54 mg/ml and a CC50 higher than 105 being obtained for lymphoblastoid (C8166) cells (41). More similar to our data, Teleshova et al. showed that PRO-2000 was not cytotoxic toward dendritic cells or CD4+ T cells at the highest concentration tested (20 μM) (33), whereas it had an EC50 of 5.8 μM in cervical tissue explants (14). Next, GML provided promising results in a simian immunodeficiency virus-macaque model, inhibiting a mucosal signaling pathway that resulted in the protection of rhesus macaques from simian immunodeficiency virus infection. We confirmed that GML does not induce proinflammatory IL-8, but it caused cell death and disruption of the epithelial barrier at concentrations near its active in vivo concentration.
It is clear from previous prevention studies that modifications in formulations, including changes to the presumably inactive ingredients used, can alter the efficacy and/or toxicity of a vaginal preparation (18). Therefore, in our toxicity assays we assessed the toxicity profiles of individual excipients belonging to different classes. This would allow formulation scientists to choose the most appropriate excipients. As various excipients belonging to various classes are added to a formulation, the present toxicity data may constitute useful guidance to select the most promising excipients displaying the least toxicity in vitro (i.e., a typical concentration below the CC50).
Various excipients decreased viability and/or the epithelial layer integrity of HEC-1A cells at concentration levels that may be used in pharmaceutical preparations. Within each class of excipients, a ranking of the toxicity of the excipients could be made. This high level of toxicity observed with the excipients raises the question whether the used models are not too sensitive compared to the in vivo situation. The absence of an in vivo “gold standard” for safety testing precludes the evaluation of the biorelevance of the present model. However, as already mentioned, the results of API testing suggest that the sensitivity of our model is similar to the sensitivities of other conventional in vitro systems (i.e., CC50 values are in the same range) (13, 34, 38). Moreover, comparing values obtained in the various viability assays, the CC50 values obtained in the cervical explant model, which was used for comparative reasons, were between the values obtained in the nonpolarized and the polarized exposure setups with HEC-1A cells.
Significant correlations were found between the data obtained in the various assays. This implies that the different assays do not generate independent data, although the sensitivities can differ. Therefore, we suggest the use of the simplest assay (nonpolarized exposure) for a first high-throughput screening of candidate compounds. Promising compounds could then be confirmed in a more relevant model, such as the dual-chamber model.
Discrepancies between in vitro findings (e.g., our results with PRO-2000) and the results of the clinical trials (e.g., the results of the phase I trial with PRO-2000) may be due to various factors. On the one hand, the settings of preclinical testing and clinical trials are different in terms of the microbicide concentration and length of exposure to the microbicides used. On the other hand, the end points of clinical safety trials are different and include histopathologic/microbiologic measures, the inflammation of the genital mucosa assessed, and the impact on the vaginal pH and microflora determined. Moreover, epithelial layer permeability, which is an important parameter reflecting the barrier function of the epithelium against HIV (15), is not assessed in human safety trials. The sensitivity to detect possible changes in cell viability and epithelial layer integrity using colposcopy is low due to the regeneration capacity of the epithelium and the microflora. However, small transient changes that are undetectable by colposcopy could already increase the susceptibility to HIV. Therefore, as long as there is no certainty about the in vivo standard for safety assessment, potential safety issues detected in in vitro/ex vivo assays should be seriously considered. The international efforts to identify vaginal biomarkers, including cytokines and chemokines, are the way forward to correlate in vitro and in vivo safety testing more properly.
In this context, a correlation between mucosal toxicity and increased levels of the proinflammatory chemokine IL-8 in vaginal washings of spermicide-treated rabbits has been observed (12). The significant increase of IL-8 observed upon treatment with noncytotoxic concentrations of some APIs and excipients might result in an increased susceptibility to HIV infection in vivo, because IL-8 might attract HIV target cells. Therefore, assessment of IL-8 might be used as a sensitive assay complementary to the existing in vitro toxicity and epithelial layer integrity assays.
Recently, Rohan et al. showed that a tenofovir gel and a placebo gel composed of hydroxyethycellulose, EDTA, citric acid, glycerin, and the preservatives methyl- and propylparaben were detrimental toward epithelial cells and explants, causing a reduced viability and epithelial layer integrity of HEC-1A and Caco-2 cells and cervical tissue explants (30). Similar observations were made by Dezzutti et al., assessing the toxicity and epithelial layer integrity of HEC-1A and Caco-2 cell lines after exposure to PRO-2000 gel, UC-781 gel, and the placebo gels methylcellulose and Vena Gel (7). Moreover, even higher levels of toxicity were observed with KY jelly placebo gel than with Vena Gel. These data are in line with our data and suggest safety issues with the use of certain placebo gels/pharmaceutical excipients.
In the present study, various cell lines that originate from the upper and lower female genital tract were used, and only slight differences in sensitivity were observed, although the CaSki cell line forms a monolayer, whereas HEC-1A and SiHa cells tend to form stratified epithelia with a thickness of 4 to 5 cell layers (15). The HEC-1A cell model might therefore not be very representative of the uterine (or endocervical) epithelia, which are monolayers, but rather might represent an intermediate between the endo- and ectocervical epithelia. Although it is possible that the toxicity tested in a model of truly multistratified epithelium may be lower, APIs and excipients should not be deleterious toward single-layered epithelia. In fact, ectopy, a condition which is characterized by the extension of endocervical single-layered columnar epithelium over the ectocervix, is a frequently occurring nonpathological condition present in adolescent women around the menarche, during pregnancy, and postpartum. Because young women are most vulnerable for HIV infection, an effective microbicide designed to prevent the further spread of HIV, especially in Africa, should be tailored to this specific population (25, 28). Therefore, each product (API or excipient) that causes the slightest damage to this ectopic endocervix is unwanted.
In summary, a high-throughput dual-chamber model has been evaluated for microbicide safety testing. Surprisingly, cell viability and epithelial layer integrity were compromised by most excipients at concentrations near the typically used concentration. A toxicity ranking of the excipients was made within each class. IL-8 was induced by several excipients at subtoxic concentrations, suggesting that even low concentrations of some compounds may induce a proinflammatory environment. Among the active ingredients, UC-781 and PMPA showed no toxicity and almost no induction of IL-8 at the tested concentrations, whereas TMC-120, PRO-2000, GML, and N-9 showed some toxic effects and/or induced IL-8 secretion. Early identification of safety issues concerning the use of pharmaceutical excipients should reduce the risk of toxicity failure in animal and clinical testing of microbicide preparations.
Acknowledgments
Youssef Gali is a predoctoral fellow of the Institute for Science and Technology (IWT). This work was supported by the Research Foundation—Flanders (Belgium) (grant G.0125.06) and the Agence Nationale de Recherches sur le Sida (ANRS). We are grateful to the Dormeur Foundation for supporting our research by funding our TriStar fluorometer. We thank the EUROPRISE Network of Excellence for support.
Special thanks go to Marijke Trog, Ellen Van Holle, Marleen Temmerman, and Marleen Praet from Ghent University Hospital and to the patients for donating cervical tissue.
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Adler, K., T. Erickson, and M. Bobrow. 1997. High sensitivity detection of HPV-16 in SiHa and CaSki cells utilizing FISH enhanced by TSA. Histochem. Cell Biol. 108:321-324. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. C., W. C. Phelps, V. Lindgren, M. J. Braun, M. A. Gonda, and P. M. Howley. 1987. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 61:962-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer, B. E., G. F. Doncel, F. C. Krebs, R. J. Shattock, P. S. Fletcher, R. W. Buckheit, Jr., K. Watson, C. S. Dezzutti, J. E. Cummins, E. Bromley, N. Richardson-Harman, L. A. Pallansch, C. Lackman-Smith, C. Osterling, M. Mankowski, S. R. Miller, B. J. Catalone, P. A. Welsh, M. K. Howett, B. Wigdahl, J. A. Turpin, and P. Reichelderfer. 2006. In vitro preclinical testing of nonoxynol-9 as potential anti-human immunodeficiency virus microbicide: a retrospective analysis of results from five laboratories. Antimicrob. Agents Chemother. 50:713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blocker, M. E., and M. S. Cohen. 2000. Biologic approaches to the prevention of sexual transmission of human immunodeficiency virus. Infect. Dis. Clin. North Am. 14:983-999. [DOI] [PubMed] [Google Scholar]
- 5.Cummins, J. E., Jr., J. Guarner, L. Flowers, P. C. Guenthner, J. Bartlett, T. Morken, L. A. Grohskopf, L. Paxton, and C. S. Dezzutti. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother. 51:1770-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler, B., and J. Justman. 2008. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect. Dis. 8:685-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias, C. J., and C. Coggins. 1996. Female-controlled methods to prevent sexual transmission of HIV. AIDS 10(Suppl 3):S43-S51. [PubMed] [Google Scholar]
- 9.FDA. Inactive ingredient search for approved drug products. FDA, Washington, DC. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm.
- 10.Feldblum, P. J., A. Adeiga, R. Bakare, S. Wevill, A. Lendvay, F. Obadaki, M. O. Olayemi, L. Wang, K. Nanda, and W. Rountree. 2008. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One 3:e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichorova, R. N., M. Bajpai, N. Chandra, J. G. Hsiu, M. Spangler, V. Ratnam, and G. F. Doncel. 2004. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol. Reprod. 71:761-769. [DOI] [PubMed] [Google Scholar]
- 12.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9 induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher, P., S. Harman, H. Azijn, N. Armanasco, P. Manlow, D. Perumal, M. P. de Bethune, J. Nuttall, J. Romano, and R. Shattock. 2009. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 53:487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher, P. S., G. S. Wallace, P. M. Mesquita, and R. J. Shattock. 2006. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology 3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gali, Y., K. K. Arien, M. Praet, R. Van den Bergh, M. Temmerman, O. Delezay, and G. Vanham. 2010. Development of an in vitro dual-chamber model of the female genital tract as a screening tool for epithelial toxicity. J. Virol. Methods 165:186-197. [DOI] [PubMed] [Google Scholar]
- 16.Garg, S., K. R. Tambwekar, K. Vermani, A. Garg, C. L. Kaul, and L. J. D. Zaneveld. 2001. Compendium of pharmaceutical excipients for vaginal formulations. Pharm. Technol. 25:14-24. [Google Scholar]
- 17.Garg, S., K. R. Tambwekar, K. Vermani, R. Kandarapu, A. Garg, D. P. Waller, and L. J. Zaneveld. 2003. Development pharmaceutics of microbicide formulations. Part II. Formulation, evaluation, and challenges. AIDS Patient Care STDs 17:377-399. [DOI] [PubMed] [Google Scholar]
- 18.Goeman, J., I. Ndoye, L. M. Sakho, S. Mboup, P. Piot, M. Karam, E. Belsey, J. M. Lange, M. Laga, and J. H. Perriens. 1995. Frequent use of menfegol spermicidal vaginal foaming tablets associated with a high incidence of genital lesions. J. Infect. Dis. 171:1611-1614. [DOI] [PubMed] [Google Scholar]
- 19.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halpern, V., F. Ogunsola, O. Obunge, C.-H. Wang, N. Onyejepu, O. Oduyebo, D. Taylor, L. McNeil, N. Mehta, J. Umo-Otong, S. Otusanya, T. Crucitti, and S. Abdellati. 2008. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a phase III trial in Nigeria. PLoS One 3:e3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillier, S. L., T. Moench, R. Shattock, R. Black, P. Reichelderfer, and F. Veronese. 2005. In vitro and in vivo: the story of nonoxynol 9. J. Acquir. Immune Defic. Syndr. 39:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Krebs, F. C., S. R. Miller, B. J. Catalone, R. Fichorova, D. Anderson, D. Malamud, M. K. Howett, and B. Wigdahl. 2002. Comparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrob. Agents Chemother. 46:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuramoto, H., S. Tamura, and Y. Notake. 1972. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am. J. Obstet. Gynecol. 114:1012-1019. [DOI] [PubMed] [Google Scholar]
- 24.Mauck, C. K., D. H. Weiner, M. D. Creinin, K. T. Barnhart, M. M. Callahan, and R. Bax. 2004. A randomized phase I vaginal safety study of three concentrations of C31G vs. extra strength Gynol II. Contraception 70:233-240. [DOI] [PubMed] [Google Scholar]
- 25.Mayer, K. H., and D. J. Anderson. 1995. Heterosexual HIV transmission. Infect. Agents Dis. 4:273-284. [PubMed] [Google Scholar]
- 26.Mayer, K. H., S. A. Karim, C. Kelly, L. Maslankowski, H. Rees, A. T. Profy, J. Day, J. Welch, and Z. Rosenberg. 2003. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS 17:321-329. [DOI] [PubMed] [Google Scholar]
- 27.Mesquita, P. M., N. Cheshenko, S. S. Wilson, M. Mhatre, E. Guzman, E. Fakioglu, M. J. Keller, and B. C. Herold. 2009. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J. Infect. Dis. 200:599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss, G. B., D. Clemetson, L. D'Costa, F. A. Plummer, J. O. Ndinya-Achola, M. Reilly, K. K. Holmes, P. Piot, G. M. Maitha, S. L. Hillier, et al. 1991. Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: results of a study of couples in Nairobi, Kenya. J. Infect. Dis. 164:588-591. [DOI] [PubMed] [Google Scholar]
- 29.Roehr, B. 2009. Microbicide offers no protection against HIV infection. BMJ 339:b5538. [DOI] [PubMed] [Google Scholar]
- 30.Rohan, L. C., B. J. Moncla, R. P. Kunjara Na Ayudhya, M. Cost, Y. Huang, F. Gai, N. Billitto, J. D. Lynam, K. Pryke, P. Graebing, N. Hopkins, J. F. Rooney, D. Friend, and C. S. Dezzutti. 2010. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 5:e9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlievert, P. M., K. L. Strandberg, A. J. Brosnahan, M. L. Peterson, S. E. Pambuccian, K. R. Nephew, K. G. Brunner, N. J. Schultz-Darken, and A. T. Haase. 2008. Glycerol monolaurate does not alter rhesus macaque (Macaca mulatta) vaginal lactobacilli and is safe for chronic use. Antimicrob. Agents Chemother. 52:4448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoler-Karpoff, S., G. Ramjee, K. Ahmed, L. Altini, M. G. Plagianos, B. Friedland, S. Govender, A. De Kock, N. Cassim, T. Palanee, G. Dozier, R. Maguire, and P. Lahteenmaki. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977-1987. [DOI] [PubMed] [Google Scholar]
- 33.Teleshova, N., T. Chang, A. Profy, and M. E. Klotman. 2008. Inhibitory effect of PRO 2000, a candidate microbicide, on dendritic cell-mediated human immunodeficiency virus transfer. Antimicrob. Agents Chemother. 52:1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terrazas-Aranda, K., Y. Van Herrewege, D. Hazuda, P. Lewi, R. Costi, R. Di Santo, A. Cara, and G. Vanham. 2008. Human immunodeficiency virus type 1 (HIV-1) integration: a potential target for microbicides to prevent cell-free or cell-associated HIV-1 infection. Antimicrob. Agents Chemother. 52:2544-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. M. Tshibaka, V. Ettiègne-Traoré, C. Uaheowitchai, S. S. A. Karim, B. Mâsse, J. Perriëns, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 36.Van der Straten, A., S. Napierala, H. Cheng, C. Mauck, T. Depineres, P. Dhlakama, M. Thompson, T. Chipato, N. Hammond, and N. Padian. 2007. A randomized controlled safety trial of the diaphragm and cellulose sulfate microbicide gel in sexually active women in Zimbabwe. Contraception 76:389-399. [DOI] [PubMed] [Google Scholar]
- 37.Van de Wijgert, J. H., S. L. Braunstein, N. S. Morar, H. E. Jones, L. Madurai, T. T. Strickfaden, M. Moodley, J. Aboobaker, G. Ndlovu, T. M. Ferguson, B. A. Friedland, C. E. Hart, and G. Ramjee. 2007. Carraguard vaginal gel safety in HIV-positive women and men in South Africa. J. Acquir. Immune Defic. Syndr. 46:538-546. [DOI] [PubMed] [Google Scholar]
- 38.Van Herrewege, Y., J. Michiels, A. Waeytens, G. De Boeck, E. Salden, L. Heyndrickx, G. van den Mooter, M. P. de Bethune, K. Andries, P. Lewi, M. Praet, and G. Vanham. 2007. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Res. 74:111-124. [DOI] [PubMed] [Google Scholar]
- 39.Vermani, K., and S. Garg. 2000. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 3:359-364. [DOI] [PubMed] [Google Scholar]
- 40.Weber, J., K. Desai, and J. Darbyshire. 2005. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med. 2:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber, J., A. Nunn, T. O'Connor, D. Jeffries, V. Kitchen, S. McCormack, J. Stott, N. Almond, A. Stone, and J. Darbyshire. 2001. ‘Chemical condoms’ for the prevention of HIV infection: evaluation of novel agents against SHIV(89.6PD) in vitro and in vivo. AIDS 15:1563-1568. [DOI] [PubMed] [Google Scholar]