Abstract
Resistance of Gram-positive pathogens to first-line antimicrobial agents has been increasing in many parts of the world. We compared the in vitro activities of torezolid with those of other antimicrobial agents, including linezolid, against clinical isolates of major aerobic and anaerobic bacteria. Torezolid had an MIC90 of ≤0.5 μg/ml for the Gram-positive bacterial isolates tested and was more potent than either linezolid or vancomycin.
Antimicrobial resistance in Gram-positive cocci has become a major problem in recent years. Oxazolidinones, a new therapeutic class of synthetic drugs, are active against Gram-positive pathogens. Linezolid, the only marketed oxazolidinone, inhibits the initiation of bacterial protein translation by binding to the 23S rRNA peptidyl transferase region (15). The widely used drug linezolid is effective against most Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp., and penicillin-resistant Streptococcus pneumoniae (1, 2). However, several recent studies have reported the emergence of linezolid-resistant staphylococci and enterococci in Brazil, China, France, Germany, Italy, and Sweden. The dominant resistance mechanisms are mutations of the 23S rRNA gene and the recently described mobile chloramphenicol-florfenicol resistance (cfr) methyltransferase gene (9).
The antibacterial activity of oxazolidinones depends on their affinity for the site of action on the ribosome. Therefore, by modifying their chemical structure, novel oxazolidinones with improved antimicrobial activity can be obtained. Accordingly, it is important to find more useful and less toxic oxazolidinones. Torezolid [TR-700, DA-7157; R-3-(4-(2-(2-methyltetrazol-5-yl)pyridine-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-on] is the active moiety of the prodrug torezolid phosphate (TR-701, DA-7218) (Fig. 1). In a recent study, torezolid was 4- to 8-fold more active than linezolid against Gram-positive bacteria collected from the United States (3). In another study, torezolid demonstrated an 8- to 16-fold increase in potency against all of the linezolid-resistant isolates tested, including MRSA, MRSA carrying the mobile cfr methyltransferase gene, and vancomycin-resistant enterococci (14). However, as far as we know, the activities of torezolid against anaerobic bacteria have not been reported.
FIG. 1.
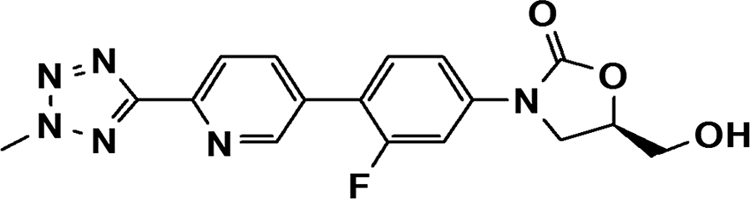
Chemical structure of torezolid.
Human plasma protein binding of torezolid was about 80% (data not shown), and the MIC was unaffected by the presence of 20% human plasma (4). Torezolid has a better pharmacokinetic profile than linezolid. After oral administration of torezolid at 200 mg once a day, the maximum concentration of the drug in serum, half-life, and area under the curve were 2.0 μg/ml, 11.2 h, and 25.4 μg·h/ml, respectively (13). In another study, torezolid phosphate was safe and effective with once-daily 200-mg dosing over 5 to 7 days of treatment for severe complicated skin and skin structure infections caused by Gram-positive bacteria (16). In this study, we compared the in vitro activities of torezolid with those of other antimicrobial agents, including linezolid, against clinical isolates of major aerobic and anaerobic Gram-positive and Gram-negative bacteria.
(Part of this study was presented at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2004 [12]).
Five hundred ten nonduplicate aerobic and anaerobic bacterial isolates were collected between 2002 and 2004 from patients at a South Korean tertiary-care hospital. The species were identified by conventional methods or by using either the ID 32 GN or the ATB 32A system (bioMérieux, Marcy-l'Etoile, France). Antimicrobial susceptibility was tested by the CLSI agar dilution method (5, 6, 7). The media used were Mueller-Hinton agar (Becton Dickinson, Sparks, MD) for testing of Staphylococcus spp., Enterococcus spp., and Moraxella catarrhalis; Mueller-Hinton agar supplemented with 5% sheep blood for Streptococcus spp.; Haemophilus test medium for Haemophilus influenzae; and brucella agar (Becton Dickinson) supplemented with 5 μg hemin, 1 μg vitamin K1 per ml, and 5% laked sheep blood for anaerobic bacteria.
The antimicrobial agents used were torezolid and linezolid (Dong-A, Seoul, South Korea); erythromycin, tetracycline, oxacillin, penicillin G, and cefuroxime (Sigma Chemical, St. Louis, MO); piperacillin and tazobactam (Yuhan, Seoul, South Korea); azithromycin and sulbactam (Pfizer Korea, Seoul, South Korea); clindamycin (Korea Upjohn, Seoul, South Korea); levofloxacin (Daiichi, Tokyo, Japan); ampicillin, gentamicin, and chloramphenicol (Chong Kun Dang, Seoul, South Korea); cefotaxime (Han-Dok, Seoul, South Korea); cefoxitin and imipenem (Merck Sharp & Dohme, Rahway, NJ); cefotetan (Je Il, Seoul, South Korea); metronidazole (Choong Wae, Seoul, South Korea); trimethoprim and sulfamethoxazole (Dong Wha, Seoul, South Korea); cefaclor and vancomycin (Daewoong, Seoul, South Korea); and teicoplanin (Sanofi Aventis, Bridgewater, NJ).
American Type Culture Collection strains of S. aureus (ATCC 29213), Enterococcus faecalis (ATCC 29212), S. pneumoniae (ATCC 49619), H. influenzae (ATCC 49247), Bacteroides fragilis (ATCC 25285), and Bacteroides thetaiotaomicron (ATCC 29741) were used as reference strains. The meningeal breakpoints of penicillin G and cefotaxime were used for S. pneumoniae.
MRSA continues to be prevalent in South Korea, accounting for 64% of the S. aureus strains in one study (10). In this study, all of the isolates of staphylococci tested were inhibited by torezolid at ≤1 μg/ml and the MIC for 90% of the strains tested (MIC90) was 4- to 8-fold lower than that of linezolid (Table 1). The majority of the MRSA isolates was resistant to erythromycin, clindamycin, gentamicin, levofloxacin, and tetracycline.
TABLE 1.
Comparative antimicrobial activities of torezolid and other antimicrobial agents against aerobic and anaerobic bacteria
| Organism (no. of isolates tested) and antimicrobial agent | Breakpoint (μg/ml)f |
MIC (μg/ml) |
Susceptibility (%)f |
||||||
|---|---|---|---|---|---|---|---|---|---|
| S | I | R | Range | 50% | 90% | S | I | R | |
| Methicillin-susceptible S. aureus (30) | |||||||||
| Torezolid | NAg | NA | NA | 0.5-1 | 0.5 | 0.5 | NA | NA | NA |
| Linezolid | ≤4 | ≥8 | 2-4 | 4 | 4 | 100 | NA | 0 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 0.5->128 | 0.5 | >128 | 63 | 7 | 30 |
| Clindamycin | ≤0.5 | 1-2 | ≥4 | ≤0.06-1 | 0.25 | 0.25 | 97 | 3 | 0 |
| Cotrimoxazole | ≤2/38 | ≥4/76 | ≤0.06-32 | 0.25 | 2 | 90 | NA | 10 | |
| Gentamicin | ≤4 | 8 | ≥16 | 0.06->128 | 0.5 | 128 | 70 | 3 | 27 |
| Levofloxacin | ≤1 | 2 | ≥4 | 0.5-8 | 0.5 | 1 | 97 | 0 | 3 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.25-64 | 0.5 | 32 | 83 | 0 | 17 |
| Oxacillin | ≤2 | ≥4 | 0.06-0.5 | 0.5 | 0.5 | 100 | NA | 0 | |
| Vancomycin | ≤2 | 4-8 | ≥16 | 0.5-1 | 0.5 | 1 | 100 | 0 | 0 |
| MRSA (30) | |||||||||
| Torezolid | NA | NA | NA | 0.5 | 0.5 | 0.5 | NA | NA | NA |
| Linezolid | ≤4 | ≥8 | 2-4 | 2 | 4 | 100 | NA | 0 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 0.5->128 | >128 | >128 | 3 | 3 | 93 |
| Clindamycin | ≤0.5 | 1-2 | ≥4 | 0.25->128 | >128 | >128 | 17 | 0 | 83 |
| Cotrimoxazole | ≤2/38 | ≥4/76 | 0.25->128 | 0.5 | >128 | 73 | NA | 27 | |
| Gentamicin | ≤4 | 8 | ≥16 | 0.25->128 | 64 | >128 | 13 | 0 | 87 |
| Levofloxacin | ≤1 | 2 | ≥4 | 0.5->128 | 16 | >128 | 17 | 0 | 83 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.5-128 | 64 | 64 | 33 | 0 | 67 |
| Oxacillin | ≤2 | ≥4 | 32->128 | >128 | >128 | 0 | NA | 100 | |
| Vancomycin | ≤2 | 4-8 | ≥16 | 0.5-2 | 1 | 1 | 100 | 0 | 0 |
| Methicillin-susceptible, coagulase negative staphylococci (29) | |||||||||
| Torezolid | NA | NA | NA | 0.25-0.5 | 0.5 | 0.5 | NA | NA | NA |
| Linezolid | ≤4 | ≥8 | 1-4 | 2 | 4 | 100 | NA | 0 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 0.25->128 | 0.5 | 128 | 76 | 0 | 24 |
| Clindamycin | ≤0.5 | 1-2 | ≥4 | 0.12->128 | 0.25 | 1 | 90 | 7 | 3 |
| Cotrimoxazole | ≤2/38 | ≥4/76 | ≤0.06-32 | 0.25 | 16 | 90 | NA | 10 | |
| Gentamicin | ≤4 | 8 | ≥16 | 0.06-128 | 0.12 | 64 | 69 | 7 | 24 |
| Levofloxacin | ≤1 | 2 | ≥4 | 0.25-32 | 0.5 | 0.5 | 97 | 0 | 3 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.5-128 | 0.5 | 32 | 76 | 0 | 24 |
| Oxacillin | ≤0.25 | ≥0.5 | 0.06-0.25 | 0.12 | 0.25 | 100 | NA | 0 | |
| Vancomycin | ≤2 | 4-8 | ≥16 | 0.5-2 | 1 | 1 | 100 | 0 | 0 |
| Methicillin-resistant, coagulase negative staphylococci (26) | |||||||||
| Torezolid | NA | NA | NA | 0.12-0.5 | 0.5 | 0.5 | NA | NA | NA |
| Linezolid | ≤4 | ≥8 | 0.5-4 | 2 | 2 | 100 | NA | 0 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | ≤0.06->128 | 64 | 128 | 42 | 0 | 58 |
| Clindamycin | ≤0.5 | 1-2 | ≥4 | 0.12->128 | 0.25 | >128 | 62 | 0 | 38 |
| Cotrimoxazole | ≤2/38 | ≥4/76 | ≤0.06-32 | 2 | 32 | 50 | NA | 50 | |
| Gentamicin | ≤4 | 8 | ≥16 | 0.06-128 | 16 | 64 | 27 | 15 | 58 |
| Levofloxacin | ≤1 | 2 | ≥4 | 0.12-16 | 0.5 | 16 | 73 | 12 | 15 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.5->128 | 4 | 128 | 69 | 4 | 27 |
| Oxacillin | ≤0.25 | ≥0.5 | 0.5->128 | 4 | 64 | 0 | NA | 100 | |
| Vancomycin | ≤2 | 4-8 | ≥16 | 0.25-2 | 1 | 2 | 100 | 0 | 0 |
| Vancomycin-susceptible Enterococcus faecalis (49) | |||||||||
| Torezolid | NA | NA | NA | 0.12-0.5 | 0.25 | 0.5 | NA | NA | NA |
| Linezolid | ≤2 | 4 | ≥8 | 0.5-2 | 2 | 2 | 100 | 0 | 0 |
| Ampicillin | ≤8 | ≥16 | 0.25-8 | 1 | 4 | 100 | NA | 0 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 0.12->128 | 4 | >128 | 9 | 42 | 49 |
| Levofloxacin | ≤2 | 4 | ≥8 | 0.5-64 | 2 | 64 | 69 | 0 | 31 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.5-128 | 64 | 64 | 20 | 0 | 80 |
| Vancomycin | ≤4 | 8-16 | ≥32 | 1-4 | 2 | 2 | 100 | 0 | 0 |
| Teicoplanin | ≤8 | 16 | ≥32 | ≤0.12-0.5 | 0.25 | 0.5 | 100 | 0 | 0 |
| Vancomycin-resistant E. faecalis (12) | |||||||||
| Torezolid | NA | NA | NA | 0.25-0.5 | 0.25 | 0.5 | NA | NA | NA |
| Linezolid | ≤2 | 4 | ≥8 | 0.5-1 | 1 | 1 | 100 | 0 | 0 |
| Ampicillin | ≤8 | ≥16 | 1-4 | 2 | 4 | 100 | NA | 0 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | >128 | >128 | >128 | 0 | 0 | 100 |
| Levofloxacin | ≤2 | 4 | ≥8 | 16-128 | 64 | 64 | 0 | 0 | 100 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.5-64 | 32 | 64 | 8 | 0 | 92 |
| Vancomycin | ≤4 | 8-16 | ≥32 | >128 | >128 | >128 | 0 | 0 | 100 |
| Teicoplanin | ≤8 | 16 | ≥32 | 32-128 | 64 | 64 | 0 | 0 | 100 |
| Vancomycin-susceptible Enterococcus faecium (30) | |||||||||
| Torezolid | NA | NA | NA | 0.06-0.25 | 0.25 | 0.25 | NA | NA | NA |
| Linezolid | ≤2 | 4 | ≥8 | 0.5-2 | 2 | 2 | 100 | 0 | 0 |
| Ampicillin | ≤8 | ≥16 | 1->128 | >128 | >128 | 7 | NA | 93 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 0.25->128 | >128 | >128 | 3 | 7 | 90 |
| Levofloxacin | ≤2 | 4 | ≥8 | 2-128 | 64 | 64 | 3 | 7 | 90 |
| Tetracycline | ≤4 | 8 | ≥16 | 0.12-32 | 0.5 | 1 | 97 | 0 | 3 |
| Vancomycin | ≤4 | 8-16 | ≥32 | 0.5-4 | 0.5 | 0.5 | 100 | 0 | 0 |
| Teicoplanin | ≤8 | 16 | ≥32 | 0.25-2 | 0.5 | 0.5 | 100 | 0 | 0 |
| Vancomycin-resistant E. faecium (29) | |||||||||
| Torezolid | NA | NA | NA | 0.06-0.25 | 0.12 | 0.25 | NA | NA | NA |
| Linezolid | ≤2 | 4 | ≥8 | 0.5-1 | 1 | 1 | 100 | 0 | 0 |
| Ampicillin | ≤8 | ≥16 | 64->128 | >128 | >128 | 0 | NA | 100 | |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 64->128 | 128 | >128 | 0 | 0 | 100 |
| Levofloxacin | ≤2 | 4 | ≥8 | 16-128 | 64 | 128 | 0 | 0 | 100 |
| Tetracycline | ≤4 | 8 | ≥16 | ≤0.06-128 | 0.25 | 128 | 90 | 0 | 10 |
| Vancomycin | ≤4 | 8-16 | ≥32 | 64->128 | 128 | >128 | 0 | 0 | 100 |
| Teicoplanin | ≤8 | 16 | ≥32 | 2-64 | 16 | 64 | 21 | 31 | 48 |
| S. pneumoniae (29) | |||||||||
| Torezolid | NA | NA | NA | 0.12-0.5 | 0.25 | 0.25 | NA | NA | NA |
| Linezolid | ≤2 | 0.5-2 | 1 | 1 | 100 | NA | NA | ||
| Penicillin G | ≤0.06 | ≥0.12 | 0.015-2 | 1 | 2 | 17 | NA | 83 | |
| Cefotaximec | ≤0.5 | 1 | ≥2 | 0.015-2 | 1 | 2 | 31 | 55 | 14 |
| Clindamycin | ≤0.25 | 0.5 | ≥1 | 0.25->128 | >128 | >128 | 28 | 0 | 72 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | 0.25->128 | >128 | >128 | 14 | 0 | 86 |
| Cotrimoxazole | ≤0.5/9.5 | 1/19-2/38 | ≥4/76 | 0.5-128 | 16 | 64 | 24 | 10 | 66 |
| Levofloxacin | ≤2 | 4 | ≥8 | 1-2 | 2 | 2 | 100 | 0 | 0 |
| Tetracycline | ≤2 | 4 | ≥8 | ≤0.12-32 | 16 | 32 | 10 | 0 | 90 |
| S. pyogenes (15) | |||||||||
| Torezolid | NA | NA | NA | 0.06-0.25 | 0.12 | 0.25 | NA | NA | NA |
| Linezolid | ≤2 | 1-2 | 1 | 2 | 100 | NA | NA | ||
| Penicillin G | ≤0.12 | ≤0.008-0.015 | 0.015 | 0.015 | 100 | NA | NA | ||
| Cefotaxime | ≤0.5 | ≤0.008-0.03 | 0.015 | 0.03 | 100 | NA | NA | ||
| Clindamycin | ≤0.25 | 0.5 | ≥1 | 0.12-0.25 | 0.12 | 0.25 | 100 | 0 | 0 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | 0.12-0.25 | 0.12 | 0.25 | 100 | 0 | 0 |
| Levofloxacin | ≤2 | 4 | ≥8 | 0.5-4 | 1 | 4 | 80 | 20 | 0 |
| S. agalactiae (15) | |||||||||
| Torezolid | NA | NA | NA | 0.12-0.5 | 0.25 | 0.5 | NA | NA | NA |
| Linezolid | ≤2 | 1-2 | 2 | 2 | 100 | NA | NA | ||
| Penicillin G | ≤0.12 | 0.03-0.06 | 0.06 | 0.06 | 100 | NA | NA | ||
| Cefotaxime | ≤0.5 | 0.03-0.06 | 0.06 | 0.06 | 100 | NA | NA | ||
| Clindamycin | ≤0.25 | 0.5 | ≥1 | 0.25->128 | 0.25 | >128 | 53 | 0 | 47 |
| Erythromycin | ≤0.25 | 0.5 | ≥1 | 0.25->128 | 0.5 | >128 | 13 | 47 | 40 |
| Levofloxacin | ≤2 | 4 | ≥8 | 1-2 | 1 | 2 | 100 | 0 | 0 |
| M. catarrhalis (27) | |||||||||
| Torezolid | NA | NA | NA | 0.5-2 | 1 | 1 | NA | NA | NA |
| Linezolid | NA | NA | NA | 2-8 | 4 | 4 | NA | NA | NA |
| Penicillin G | NA | NA | NA | 0.03-32 | 16 | 32 | NA | NA | NA |
| Cefaclor | ≤8 | 16 | ≥32 | 0.25-32 | 2 | 8 | 96 | 0 | 4 |
| Clindamycin | ≤0.5 | 1-2 | ≥4 | 1-4 | 2 | 4 | 0 | 59 | 41 |
| Erythromycin | ≤0.5 | 1-4 | ≥8 | 0.12-0.5 | 0.25 | 0.5 | 100 | 0 | 0 |
| Levofloxacin | ≤2 | 0.06 | 0.06 | 0.06 | 100 | NA | NA | ||
| Tetracycline | ≤2 | 4 | ≥8 | 0.25-16 | 0.5 | 0.5 | 96 | 0 | 4 |
| H. influenzae (25) | |||||||||
| Torezolid | NA | NA | NA | 2-16 | 2 | 4 | NA | NA | NA |
| Linezolid | NA | NA | NA | 4-16 | 8 | 16 | NA | NA | NA |
| Ampicillin | ≤1 | 2 | ≥4 | 0.5->128 | >128 | >128 | 16 | 8 | 76 |
| Ampicillin-sulbactam | ≤2/1 | ≥4/2 | 0.5-8 | 4 | 8 | 36 | NA | 64 | |
| Cefaclor | ≤8 | 16 | ≥32 | 2->128 | 4 | >128 | 60 | 0 | 40 |
| Cefuroxime | ≤4 | 8 | ≥16 | 0.25->128 | 1 | >128 | 80 | 4 | 16 |
| Cefotaxime | ≤2 | ≤0.008-0.5 | 0.03 | 0.5 | 100 | NA | NA | ||
| Azithromycin | ≤4 | 2-4 | 4 | 4 | 100 | NA | NA | ||
| Cotrimoxazole | ≤0.5/9.5 | 1/19-2/38 | ≥4/76 | ≤0.06-32 | 4 | 32 | 48 | 0 | 52 |
| Levofloxacin | ≤2 | 0.015-0.5 | 0.03 | 0.06 | 100 | NA | NA | ||
| Tetracycline | ≤2 | 4 | ≥8 | 0.25-32 | 0.5 | 8 | 84 | 4 | 12 |
| Peptostreptococcus spp. (59)a | |||||||||
| Torezolid | NA | NA | NA | 0.03-0.25 | 0.06 | 0.25 | NA | NA | NA |
| Linezolid | NA | NA | NA | 0.25-2 | 0.5 | 1 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | ≤0.06-16 | 0.12 | 1 | 90 | 2 | 8 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | ≤0.06-8 | 0.12 | 1 | 100 | 0 | 0 |
| Piperacillin | ≤32 | 64 | ≥128 | ≤0.06-16 | ≤0.06 | 8 | 100 | 0 | 0 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | ≤0.06-16 | ≤0.06 | 8 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | ≤0.06-16 | 0.25 | 4 | 100 | 0 | 0 |
| Cefotetan | ≤16 | 32 | ≥64 | ≤0.06-128 | 0.5 | 16 | 92 | 2 | 7 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-1 | ≤0.06 | 0.12 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | ≤0.06->128 | 0.5 | 64 | 80 | 0 | 20 |
| Metronidazole | ≤8 | 16 | ≥32 | ≤0.06-4 | 1 | 2 | 100 | 0 | 0 |
| Vancomycin | NA | NA | NA | ≤0.12-1 | 0.25 | 0.5 | NA | NA | NA |
| Clostridium perfringens (15) | |||||||||
| Torezolid | NA | NA | NA | 0.12-0.25 | 0.25 | 0.25 | NA | NA | NA |
| Linezolid | NA | NA | NA | 1-2 | 2 | 2 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | ≤0.06-0.5 | ≤0.06 | 0.12 | 100 | 0 | 0 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | ≤0.06-0.5 | ≤0.06 | 0.25 | 100 | 0 | 0 |
| Piperacillin | ≤32 | 64 | ≥128 | ≤0.06-1 | ≤0.06 | 0.25 | 100 | 0 | 0 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | ≤0.06 | ≤0.06 | ≤0.06 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | 0.25-1 | 0.5 | 1 | 100 | 0 | 0 |
| Cefotetan | ≤16 | 32 | ≥64 | ≤0.06-0.5 | ≤0.06 | 0.12 | 100 | 0 | 0 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-0.12 | ≤0.06 | ≤0.06 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | ≤0.06-2 | 1 | 2 | 100 | 0 | 0 |
| Metronidazole | ≤8 | 16 | ≥32 | 1-4 | 4 | 4 | 100 | 0 | 0 |
| Vancomycin | NA | NA | NA | 0.5-2 | 0.5 | 0.5 | NA | NA | NA |
| Other Clostridium spp. (15)b | |||||||||
| Torezolid | NA | NA | NA | ≤0.06-0.25 | 0.25 | 0.25 | NA | NA | NA |
| Linezolid | NA | NA | NA | 0.5-4 | 2 | 4 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | ≤0.06-1 | 0.25 | 1 | 87 | 13 | 0 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | ≤0.06-2 | 0.25 | 1 | 100 | 0 | 0 |
| Piperacillin | ≤32 | 64 | ≥128 | ≤0.06-16 | 1 | 8 | 100 | 0 | 0 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | ≤0.06-16 | 1 | 8 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | 0.25-128 | 8 | 64 | 60 | 0 | 40 |
| Cefotetan | ≤16 | 32 | ≥64 | ≤0.06->128 | 2 | >128 | 53 | 7 | 40 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-4 | 1 | 4 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | ≤0.06->128 | 1 | >128 | 53 | 13 | 33 |
| Metronidazole | ≤8 | 16 | ≥32 | 0.12-16 | 4 | 8 | 93 | 7 | 0 |
| Vancomycin | NA | NA | NA | 0.25-8 | 4 | 8 | NA | NA | NA |
| Other anaerobic Gram-positive bacilli (13)c | |||||||||
| Torezolid | NA | NA | NA | 0.06-0.5 | 0.06 | 0.5 | NA | NA | NA |
| Linezolid | NA | NA | NA | ≤0.06-4 | 0.5 | 2 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | ≤0.06-2 | ≤0.06 | 1 | 85 | 8 | 8 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | ≤0.06-2 | 0.12 | 1 | 100 | 0 | 0 |
| Piperacillin | ≤32 | 64 | ≥128 | ≤0.06-8 | 0.5 | 8 | 100 | 0 | 0 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | ≤0.06-8 | ≤0.06 | 8 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | ≤0.06->128 | 1 | >128 | 100 | 0 | 0 |
| Cefotetan | ≤16 | 32 | ≥64 | 0.12->128 | 4 | >128 | 62 | 8 | 31 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-2 | 0.12 | 2 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | ≤0.06-4 | ≤0.06 | 2 | 92 | 8 | 0 |
| Metronidazole | ≤8 | 16 | ≥32 | 0.25->128 | >128 | >128 | 38 | 8 | 54 |
| Vancomycin | NA | NA | NA | 0.25->32 | 0.5 | >32 | NA | NA | NA |
| B. fragilis (30) | |||||||||
| Torezolid | NA | NA | NA | 1-4 | 2 | 2 | NA | NA | NA |
| Linezolid | NA | NA | NA | 2-4 | 4 | 4 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | 16->128 | 32 | >128 | 0 | 0 | 100 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | 1-32 | 2 | 16 | 83 | 7 | 10 |
| Piperacillin | ≤32 | 64 | ≥128 | 4->256 | 32 | 256 | 53 | 17 | 30 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | 0.12-8 | 0.25 | 1 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | 4-64 | 8 | 32 | 87 | 7 | 7 |
| Cefotetan | ≤16 | 32 | ≥64 | 4-128 | 8 | 32 | 83 | 7 | 10 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-4 | 0.25 | 1 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | ≤0.06->128 | 128 | >128 | 43 | 0 | 57 |
| Metronidazole | ≤8 | 16 | ≥32 | 0.5-8 | 4 | 4 | 100 | 0 | 0 |
| B. thetaiotaomicron (15) | |||||||||
| Torezolid | NA | NA | NA | 1-2 | 2 | 2 | NA | NA | NA |
| Linezolid | NA | NA | NA | 4 | 4 | 4 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | 16->128 | 32 | >128 | 0 | 0 | 100 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | 1-32 | 1 | 32 | 73 | 13 | 13 |
| Piperacillin | ≤32 | 64 | ≥128 | 16->256 | 32 | >256 | 73 | 0 | 27 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | 2-16 | 4 | 8 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | 16-32 | 16 | 32 | 73 | 27 | 0 |
| Cefotetan | ≤16 | 32 | ≥64 | 32->128 | 128 | >128 | 0 | 13 | 87 |
| Imipenem | ≤4 | 8 | ≥16 | 0.12-2 | 0.25 | 2 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | 2->128 | 8 | >128 | 7 | 40 | 53 |
| Metronidazole | ≤8 | 16 | ≥32 | 2-4 | 4 | 4 | 100 | 0 | 0 |
| Other Bacteroides spp. (14)d | |||||||||
| Torezolid | NA | NA | NA | 1-4 | 1 | 2 | NA | NA | NA |
| Linezolid | NA | NA | NA | 1-4 | 2 | 4 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | 2->128 | >128 | >128 | 0 | 0 | 100 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | 1-32 | 8 | 32 | 57 | 29 | 14 |
| Piperacillin | ≤32 | 64 | ≥128 | 2->256 | 64 | >256 | 43 | 14 | 43 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | 2-16 | 4 | 8 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | 4-64 | 16 | 32 | 79. | 14 | 7 |
| Cefotetan | ≤16 | 32 | ≥64 | 4->128 | 64 | >128 | 29 | 14 | 57 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-2 | 0.5 | 1 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | 4->128 | >128 | >128 | 0 | 7 | 93 |
| Metronidazole | ≤8 | 16 | ≥32 | ≤0.25-4 | 4 | 4 | 100 | 0 | 0 |
| Other anaerobic Gram-negative rods (27)e | |||||||||
| Torezolid | NA | NA | NA | 0.03-4 | 0.25 | 2 | NA | NA | NA |
| Linezolid | NA | NA | NA | ≤0.12-8 | 1 | 4 | NA | NA | NA |
| Ampicillin | ≤0.5 | 1 | ≥2 | ≤0.03-128 | 1 | 64 | 22 | 33 | 44 |
| Ampicillin-sulbactam | ≤8/4 | 16/8 | ≥32/16 | ≤0.03-4 | 1 | 4 | 100 | 0 | 0 |
| Piperacillin | ≤32 | 64 | ≥128 | ≤0.06-128 | 4 | 32 | 93 | 4 | 4 |
| Piperacillin-tazobactam | ≤32/4 | 64/4 | ≥128/4 | ≤0.06-8 | ≤0.06 | 4 | 100 | 0 | 0 |
| Cefoxitin | ≤16 | 32 | ≥64 | ≤0.06-8 | 1 | 4 | 100 | 0 | 0 |
| Cefotetan | ≤16 | 32 | ≥64 | ≤0.06-32 | 2 | 16 | 93 | 7 | 0 |
| Imipenem | ≤4 | 8 | ≥16 | ≤0.06-1 | ≤0.06 | 1 | 100 | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | ≤0.06->128 | ≤0.06 | 64 | 78 | 7 | 15 |
| Metronidazole | ≤8 | 16 | ≥32 | ≤0.06-4 | 0.5 | 4 | 100 | 0 | 0 |
| Chloramphenicol | ≤8 | 16 | ≥32 | 0.5-8 | 2 | 4 | 100 | 0 | 0 |
Finegoldia magna (19 strains), Peptoniphilus asaccharolyticus (15 strains), Peptostreptococcus anaerobius (12 strains), Peptostreptococcus micros (7 strains), and Anaerococcus prevotii (6 strains).
Clostridium clostridiiforme (3 strains), C. sordellii (1 strain), C. innocuum (5 strains), C. tertium (2 strains), C. ramosum (2 strains), C. sporogenes (1 strain), and C. bifermentans (1 strain).
Bifidobacterium adolescentis (2 strains), Propionibacterium acnes (4 strains), Eubacterium lentum (3 strains), Lactobacillus acidophilus (2 strains), and Actinomyces sp. (2 strains).
Bacteroides distasonis (5 strains), B. vulgatus (7 strains), and B. ovatus (2 strains).
Prevotella bivia (6 strains), P. buccae (3 strains), P. intermedia (4 strains), P. oralis (2 strains), Fusobacterium mortiferum (3 strains), F. necrophorum (2 strains), F. varium (6 strains), and Fusobacterium sp. (1 strain).
S, susceptible; I, intermediate; R, resistant.
NA, not applicable.
Vancomycin-resistant Enterococcus faecium has become prevalent in the United States (18). The vancomycin resistance rate of E. faecium has been 20% or higher in South Korean hospitals since 2003 (10). The MIC ranges of torezolid were 0.06 to 0.25 μg/ml for all of the enterococci, including vancomycin-resistant ones, while those of linezolid were 0.5 to 2 μg/ml (Table 1), which are similar to prior reports (8, 17). All of the isolates were susceptible to linezolid.
Penicillin-nonsusceptible S. pneumoniae strains were very prevalent (69%) in South Korean hospitals in 2007, when the meningeal breakpoint was applied. In this study, most of the pneumococcal isolates tested were nonsusceptible to penicillin G or cefotaxime, but the MIC range of torezolid was 0.12 to 0.5 μg/ml and the MIC90 was 4-fold lower than that of linezolid (Table 1). All of the isolates of Streptococcus pyogenes and Streptococcus agalactiae were inhibited by torezolid at ≤0.5 μg/ml.
β-Lactamase-producing M. catarrhalis and H. influenzae were prevalent in South Korea (11). The MIC ranges of torezolid for M. catarrhalis and H. influenzae were 0.5 to 2 and 2 to 16 μg/ml, respectively. The MIC90s for both of these organisms were 4-fold lower than those of linezolid.
Intraabdominal and soft-tissue infections are often due to aerobic and anaerobic bacteria. Torezolid had excellent activity against Gram-positive anaerobes (Table 1). All of the peptostreptococci and anaerobic Gram-positive bacilli were inhibited by torezolid at ≤0.5 μg/ml, and the MIC90s for these organisms were 4- to 16-fold lower than those of linezolid. The MIC90 of torezolid, 2 μg/ml, for anaerobic Gram-negative bacilli, was slightly lower than that of linezolid, 4 μg/ml (Table 1).
In conclusion, torezolid is a new antimicrobial agent with high in vitro activity against common aerobic and anaerobic Gram-positive bacteria, including multidrug-resistant isolates. Further studies are warranted to determine the clinical utility of torezolid as a therapeutic agent.
Acknowledgments
We are grateful to Young Hee Suh for technical assistance.
Footnotes
Published ahead of print on 13 September 2010.
REFERENCES
- 1.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attassi, K., E. Hershberger, R. Alam, and M. J. Zervos. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695-698. [DOI] [PubMed] [Google Scholar]
- 3.Brown, S. D., and M. M. Traczewski. 2010. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob. Agents Chemother. 54:2063-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, S., W. Im, and J. Rhee. 2007. The new oxazolidinone, TR-700 (DA-7157): effects of pH, inoculum, serum, and media on antibacterial activity, abstr. F1-1690. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL.
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, approved guideline. M45-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th ed. Approved standard M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Eliopoulos, G. M., C. B. Wennersten, and R. C. Moellering, Jr. 2002. In vitro activity of the new oxazolidinone AZD2563 against enterococci. Antimicrob. Agents Chemother. 46:3273-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, R. N., J. E. Ross, J. M. Bell, U. Utsuki, I. Fumiaki, I. Kobaysashi, and J. D. Turnidge. 2009. Zyvox annual appraisal of potency and spectrum program: linezolid surveillance program results for 2008. Diagn. Microbiol. Infect. Dis. 65:404-413. [DOI] [PubMed] [Google Scholar]
- 10.Lee K., M. Lee, J. Lee, K. H. Roh, S. J. Kim, C. H. Lee, J. J. Kim, D. Yong, S. H. Jeong, Y. Chong, and the KONSAR Group. 2009. Antimicrobial resistance surveillance in Korea in 2007: increasing prevalence of vancomycin-resistant E. faecium, cefotaxime- and cefoxitin-resistant K. pneumoniae, and imipenem-resistant P. aeruginosa and Acinetobacter spp., abstr. P639. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis., Helsinki, Finland.
- 11.Lee, K., C. H. Lim, J. H. Cho, W. G. Lee, Y. Uh, H. J. Kim, D. Yong, Y. Chong, and the KONSAR Group. 2006. High prevalence of cefazidime-resistant Klebsiella pneumoniae and increase of imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR program in 2004. Yonsei Med. J. 47:634-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, K., J. H. Yum, D. Yong, S. H. Choi, and J. K. Rhee. 2004. Comparative in vitro activity of DA-70157, a novel oxazolidinone, against recent clinical isolates of aerobic and anaerobic bacteria, abstr. F-1419. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. Washington, DC. [DOI] [PMC free article] [PubMed]
- 13.Prokocimer, P., P. Bien, K. A. Munoz, J. Bohn, R. Wright, and C. Bethune. 2008. Human pharmacokinetics of the prodrug TR-701 and TR700, its active moiety, after multiple oral doses of 200 and 400 mg TR-701, a novel oxazolidinone, abstr. F1-2064. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC.
- 14.Shaw, K. J., S. Poppe, R. Schaadt, V. Brown-Driver, J. Finn, C. M. Pillar, D. Shinabarger, and G. Zurenko. 2008. In vitro activity of TR-700, the active moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob. Agents Chemother. 52:4442-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinabarger, D. 1999. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin. Investig. Drugs 8:1195-1202. [DOI] [PubMed] [Google Scholar]
- 16.Surber, J., P. Mehra, P. Manos, J. Kingsley, M. Mascolo, B. Heller, W. O'Riordan, R. Garcia, P. Bien, C. De Anda, and P. Prokocimer. 2009. Efficacy of TR-701 in a phase 2 randomized, double-blind study in patients with severe complicated skin and skin structure infections (cSSSI), abstr. L1-335, Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA.
- 17.Sweeney, M. T., and G. E. Zurenko. 2003. In vitro activities of linezolid combined with other antimicrobial agents against staphylococci, enterococci, pneumococci, and selected gram-negative organisms. Antimicrob. Agents Chemother. 47:1902-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treitman, A. N., P. R. Yarnold, J. Warren, and G. A. Noskin. 2005. Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002). J. Clin. Microbiol. 43:462-463. [DOI] [PMC free article] [PubMed] [Google Scholar]


