Figure 2. Structural mimicry of CD4 interaction by antibody VRC01.
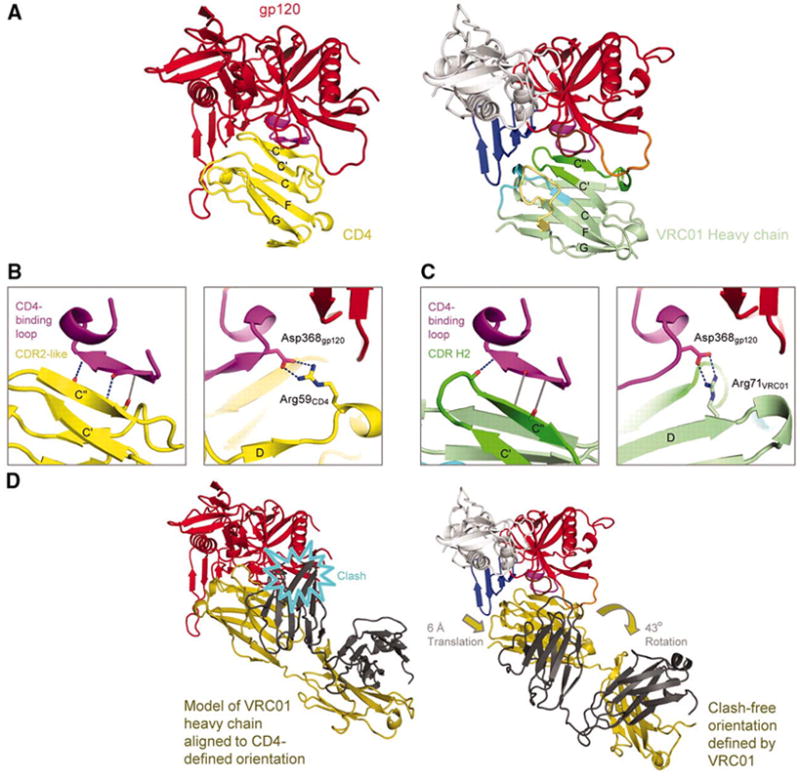
VRC01 shows how a double-headed antibody can mimic the interactions with HIV-1 gp120 of a single-headed member of the immunoglobulin superfamily such as CD4. (A) Comparison of HIV-1 gp120 binding to CD4 (N-terminal domain) and VRC01 (heavy chain-variable domain). Polypeptide chains are depicted in ribbon representation for the VRC01 complex (right) and the CD4 complex with the lowest gp120 RMSD (left) (Table S3). The CD4 complex (3JWD) (14) is colored yellow for CD4 and red for gp120, except for the CDR-binding loop (purple). The VRC01 complex is colored as in Fig. 1. Immunoglobulin domains are composed of two β-sheets, and the top sheet of both ligands is labeled with the standard immunoglobulin-strand topology (strands G, F, C, C’, C”). (B,C) Interface details for CD4 (B) and VRC01 (C). Close-ups are shown of critical interactions between the CD4-binding loop (purple) and the C” strand as well as between Asp368gp120 and either Arg59CD4 or Arg71VRC01. Hydrogen bonds with good geometry are depicted by blue dotted lines, and those with poor geometry in gray. Atoms from which hydrogen bonds extend are depicted in stick representation and colored blue for nitrogen and red for oxygen. In the left panel of C, the β15-strand of gp120 is depicted to aid comparison with B, though because of the poor hydrogen-bond geometry, it is only a loop. (D) Comparison of VRC01- and CD4-binding orientations. Polypeptides are shown in ribbon representation, with gp120 colored the same as in (A) and VRC01 depicted with heavy chain in dark yellow and light chain in dark gray. When the heavy chain of VRC01 is superimposed onto CD4 in the CD4-gp120 complex, the position assumed by the light chain evinces numerous clashes with gp120 (left). The VRC01-binding orientation (right) avoids clashes by adopting an orientation rotated by 43° and translated by 6-Å.
