Abstract
The widespread application of porcine SCNT to biomedical research is being hampered by the large adult size (300–600 lbs) of the commercial breeds commonly used for SCNT. The Yucatan minipig, in contrast, has an adult weight of 140–150 lbs and a long history of utility in biomedical research. In order to combine the wide availability of commercial swine with the biomedical value of the Yucatan minipig, we utilized SCNT using the Yucatan as nuclear donors and commercial swine as both oocyte donors and recipients. Of six recipient gilts receiving 631 SCNT embryos, three went to term and delivered seven piglets, four of which survived to adulthood. Additionally, we obtained fetal fibroblasts from a cloned Yucatan and used them for a second round of SCNT. Of three recipients receiving 315 reconstructed embryos, one went to term and delivered three piglets, one of which survived to adulthood. Both microsatellite and D-loop sequence analysis confirmed that all of the piglets generated were nuclear-mitochondrial hybrids carrying Yucatan nuclear DNA and commercial breed mitochondrial DNA. This report shows that it is possible to produce viable Yucatan SCNT clones and opens up the possibility of developing valuable biomedical models in this porcine breed.
Introduction
While cloning swine by SCNT is progressing at a rapid rate with increases in efficiency continually being reported (Estrada et al., 2007; Lai et al., 2006; Li et al., 2006; Walker et al., 2002) the incorporation of this technology into mainstream biomedical investigation will require its application to breeds of swine that are more amenable to study under a biomedical environment. The conventional occidental commercial breeds such as Yorkshire, Hampshire, Large White, Duroc, Landrace, etc., and their crossbreds have the advantage of high prolificacy, ease of management, low cost, and extensive availability. Their main drawback, however, is their large size at adulthood with intact males weighing upward of 500–600 lbs and females 400–500 lbs. This large size places severe constrains in both housing capabilities and use of animals for biomedical purposes. Of other breeds of swine available, the Yucatan has an extensive history of biomedical applications (Eubanks et al., 2006; Mattern et al., 2007; Montezuma et al., 2006; Pak et al., 2006; Panepinto et al., 1978; Svendsen, 2006; Swindle et al., 1990; Witczak et al., 2006), lines with selected SLA (swine leukocyte antigen) haplotypes for xenotransplantation have been developed (Smith et al., 2005), is considered a minipig with adult sizes of 140–150 lbs, and has a gentle disposition, making them easy to handle and manage. Unfortunately, they are expensive, costing an average of US $1,000 per animal. Moreover, their small feet size compared to conventional commercial breeds may cause problems in the post weaning to adulthood period in facilities using nonsolid flooring surfaces. In order to determine whether we could combine the strengths of both breed types, ease of availability and lower costs of the occidental commercial breeds, and the excellent biomedical properties of the Yucatan, we examined whether we could successfully clone Yucatan pigs using the occidental commercial breeds as both oocyte donors and embryo recipients, and the Yucatan pigs as the nuclear donor. Most of the previous work relating to cloning of miniature swine has been limited to in vitro development (Lee et al., 2006; Miyoshi et al., 2006). While there have been other reports of successful cloning of miniature pigs, such as the potbelly pig, the recipients utilized were either other minipigs or the Meishan breed (Hoshino et al., 2005). Recently, the birth of one Claw miniature pig using occidental breeds as oocyte donor and recipients has been reported (Miyoshi et al., 2007). However, to our knowledge, our report is the first of a successful Yucatan cloning and the second report of a successful cloning of a minipig using both oocytes and recipients from commercial occidental breeds and opens up the possibility of developing genetically modified lines of Yucatan pigs for use in biomedical research.
Materials and Methods
The experimental protocols used in this study were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Oocyte collection and maturation
Ovaries were retrieved from commercial/occidental breed sows at a slaughterhouse located 1 h from the laboratory, and transported in 0.9% saline solution at 30–35°C. Cumulus–oocyte complexes (COCs) were matured in TC199-HEPES medium supplemented with 10% porcine follicular fluid (pFF), 5 μg/mL insulin, 10 ng/mL EGF, 0.6 mM cysteine, 0.2 mM pyruvate, 25 μg/mL kanamycin and 5 IU/mL of each eCG, and hCG. Fifty COCs were cultured in 500 μL medium in a four-well Nunc dish at 38.5°C, 5% CO2 in a humidified atmosphere. COCs were cultured in this medium for 22 h before being changed to the eCG- and hCG-free culture medium for additional 16 h.
Nuclear donor cells
Yucatan nuclear donor cells were obtained from fetuses of two pregnant Yucatans at days 36 and 48 of gestation. Primary fetal porcine fibroblasts were isolated as described previously (Walker et al., 2002). Fetuses were collected in individual 50 mL centrifuge tubes containing MEM-alpha medium (Gibco, Gaithersburg, MD). Head and inner organs were excised for DNA isolation and the remnants were minced with a razor blade to less than 1 mm3 pieces. Tissue was transferred to a 15-mL tube with 10 mL 0.025% Trypsin/0.5% EDTA solution, then digested slowly while rotating (tumbling) at 37°C for 45 min. Cells were centrifuged, trypsin removed, and fresh MEM-alpha added to resuspend the pellet. Finally, cells were resuspended in 10% fetal bovine serum (FBS), 5% bovine calf serum (BCS) and 1% Penicillin-streptomycin of MEM-alpha culture medium and placed in 10-cm plates in a 37°C incubator. When plates were 70–90% confluent, cells were frozen in MEM-alpha +40% FBS + 10% DMSO, and kept in LN2 until needed for nuclear transfer. When needed, cells were thawed and grown in MEM-alpha media supplemented with 15% fetal bovine serum and incubated at 37°C in a 5% CO2 atmosphere. At 70–90% confluence, cells were removed from the plate by trypsin, centrifuged, and suspended in NCSU-23 (Petters and Wells, 1993) supplemented with 0.8 mM CaCl2 and 10% FBS. In addition, fetal fibroblasts collected as described in Estrada et al., (2007) were used as nuclear donors to generate control day 30 pregnancies.
Nuclear transfer
Oocytes collected as described above were incubated in manipulation media (Ca-free NCSU-23 with 5% FBS) containing 5 μg/mL bisbenzimide and 7.5 μg/mL cytochalasin B for 15 min.
Following this incubation period, oocytes were enucleated by removing the first polar body and metaphase II plate and one single cell was fused to each enucleated oocyte. Whenever possible, smaller sized cells were selected for SCNT. Fusion was induced by two DC pulses of 140 V for 40 μsec in 280 mM Mannitol, 0.001 mM CaCl2, and 0.05 mM MgCl2. One hour later, reconstructed oocytes were activated by two DC pulses of 110 V for 50 μsec in 280 mM Mannitol, 0.1 mM CaCl2, and 0.05 mM MgCl2 (Estrada et al., 2007). After activation, oocytes were placed back in NCSU-23 medium with 0.4% BSA and cultured at 38.5°C, 5% CO2 in a humidified atmosphere for less than 1 h before being surgically transferred into the recipient.
Recipients/foster mothers
Three-way Yorkshire × Landrace × Duroc crossbred animals from the Swine Educational Unit at NC State University were used as recipients. They were naturally cycling pigs on their first day of estrus. Gilts were checked daily for estrus detection and an ultrasound for pregnancy test was performed at day 25–30 after embryo transfer.
Microsatellite DNA analysis
Nineteen highly polymorphic microsatellites were tested to determine whether they were informative for this experiment: S0155, SW2410, SW830, S0355, SW632, SWR1941, SW936, S0228, SW857, S0101, S0143, S0178, SW911, S0002, SW980, SW240, SW175, SW951, and S0090. All were used for genotyping the donor, recipients, and clones.
Mitochondrial D-loop analysis
To determine whether any mitochondrial heteroplasmy could be detected in the Yucatan clones, and to confirm that the clones were derived from commercial breed oocytes, the D-loop region was sequenced in eight samples plus a negative control. The eight samples consisted of two of the recipient sows that gave birth to the Yucatan clones, three cloned Yucatan piglets, a recloned Yucatan cell line, and two samples from naturally bred Yucatan (not SCNT clones).
The region genotyped was selected using information derived by others (Kim et al., 2002) that identified two Yucatan specific D-loop polymorphism when compared to occidental/commercial breeds. The region of interest was sequenced using pyrosequencing technology as follows. Three primers were designed for the amplification and sequencing of the mitochondrial DNA containing the SNP of interest. For the polymerase chain reaction (PCR) a universal primer system was used. The forward primer sequence was 5′GTGACGTACTAGCAACG TCCCTGCAACCAAAACAAG3′ and the reverse primer sequence was 3′TAGCAGGATACGACTATCTGGGGACTAGCAATTAATGCAC5′. The sequence in bold is the complementary portion to the universal primers. The universal primers allowed the biotinylation of the forward sequence which was later used in the pyrosequencing process. The PCR was run in 100-μL reactions with 80 μL of Platinum® Blue PCR Supermix (Invitrogen, Carlsbad, CA), 1 μL of 5 μM gene specific primer mix, 5 μL of the 5 μM universal primer mix, 8 μL of 25 mM MgCl2, and 6 μL of 50 ng/μL template, resulting in 300 ng total of template DNA in each reaction. The thermocycler conditions used a nested PCR protocol. Initially there was a 2-min denaturation at 95°C, which was proceeded by the first cycling conditions of 94°C for 30 sec, 57°C for 15 sec, and 72°C for 15 sec for eight cycles. The second cycling conditions were 94°C for 15 sec, 58°C for 15 sec, and 72°C for 15 sec for 45 cycles. The final elongation was at 72°C for 5 min and last held at 4°C. After amplification, pyrosequencing began by the addition of 74 μL of binding buffer with 6 μL of Streptavidin Sepharose™ High-Performance beads (Amersham Biosciences, Uppsla, Sweden) to each biotinylated PCR reaction. Next, the beads were placed in wells containing 80 μL of annealing buffer with 0.32 μL of 100 μM sequencing primer 5′GGTTAAATTTTTGGGGTC3′, and the sequence analyzed with a dispensation order of GTCGATATG. The nucleotides, enzymes, and substrate from the Pyro Gold Reagents (Biotage, Uppsala, Sweden) were placed in appropriate dispensation slots according to the Sample Preparation Guidelines for the PSQ™96 and PSQ 96MA Systems.
Phenotypic characterization
Fetal and placental weights of Yucatan SCNT clones collected at day 30 of gestation were compared to similar stage SCNT clones derived from our commercial/occidental breeds. Additionally, birth weights, survival to weaning, and any detectable physical or behavioral abnormalities were recorded.
Experimental design
In this project the two questions being addressed were whether male and female Yucatan SCNT clones could be generated using oocytes and recipients from occidental breeds, and whether a second round of SCNT cloning could yield viable pregnancies. To address the first question, fibro-blasts were collected from naturally bred Yucatan gilts and the fibroblasts used for SCNT. To address the second question, pregnancies from first-generation or first-round SCNT clones were established, fetuses collected at day 30 of gestation, fetal fibroblasts cells reisolated as described above, and these fibroblasts used once again for SCNT cloning. We refer to these offspring as second-round SCNT clones. In addition, for comparison purposes, SCNT pregnancies were established using occidental breed fetal fibroblasts as nuclear donors, and pregnancies sacrificed at day 30 of gestation to obtain fetal and placental weights. For comparison of overall SCNT efficiencies, the Yucatan results were compared to our previously reported commercial breed cloning efficiencies (Estrada et al., 2007). For statistical comparisons of survival data, and fetal and placental weights data were analyzed by one-way ANOVA using SAS JMP statistical package.
Results
Fetal fibroblast collection
Two naturally mated Yucatan donors were used for development of fetal fibroblast cell lines. From one donor, four day 36 fetuses (two male and two female) were collected. Cell lines were successfully established from all fetuses and were frozen at early passages (P1 and P2) for future cloning. From the second donor, six day 48 fetuses were collected (one male and five female). Cell lines were established from all but one fetus. The failure to establish lines from one fetus was due to contamination.
To obtain fibroblasts from cloned fetuses for the second round of SCNT, 307 male and female SCNT embryos were cotransferred into three recipients all of which became pregnant. At day 30 of gestation fetuses were collected for fibroblast cell isolation, and fetal and placental weights recorded. From all recipients combined, 28 fetuses were collected—16 normal and 12 with abnormal morphology. Abnormal morphology included small size, lack of vascularization, or evidence of necrosis/resorption. Fibroblasts were collected only from fetuses with normal morphology. Overall, five female and three male, second-round SCNT cell lines were isolated. The additional eight fetuses failed to yield viable cell lines due to contamination.
SCNT
For the first round of cloning, six recipients received an average of 105 reconstructed embryos each (Table 1). Of those, three went to term. One delivered two male piglets, one of which was stillborn. The remaining clone died 7 days postnatally. Birth weight of the live clone was 1.01 kg. The second recipient delivered three female clones, all of which survived to adulthood. Birth weights were 1.12, 1.31, and 1.25 kg. The third recipient delivered three male piglets, one of which survived to adulthood (6 months of age or greater). Figure 1 shows newborn and adult Yucatan clones.
Table 1.
Efficiency of First Round and Second Round SCNT Using Yucatan Nuclear Donors
| Cloning type | Collection date | Embryos transferred (total) | Number of recipients | Pregnant at D30 (%) | Pregnant to term (%) | Live piglets born/fetuses collecteda | Survived to adulthoodb |
|---|---|---|---|---|---|---|---|
| 1st Round | Term | 631 | 6 | 3 (50%) | 3 (50%) | 7 | 4 |
| 2nd Round | Term | 315 | 3 | 1 (33%) | 1 (33%) | 3 | 1 |
| 1st Round | Day 30 | 307 | 3 | 3 (100%) | NA | 28a | NA |
Twenty-eight conceptuses 16 normal, 12 small or resorbing.
Six months of age or greater.
FIG. 1.
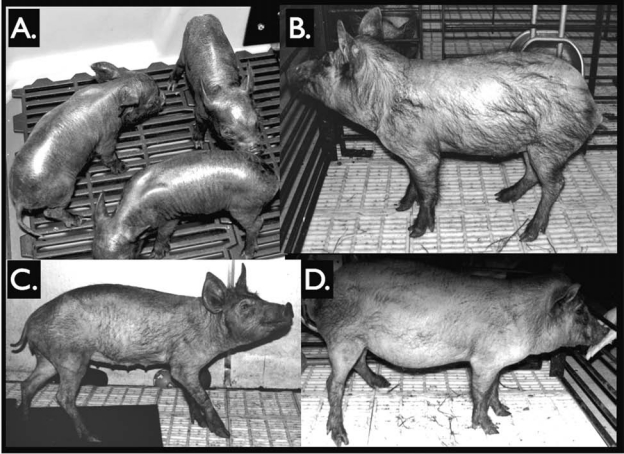
Yucatan SCNT clones. (A) Three female first-round SCNT clones at 1 week of age. (B) Adult SCNT clone boar at 11 months of age. (C) Adult SCNT clone gilt at 1 year of age. (D) Young boar from second-round SCNT (clone of clone) at 6 months of age.
For the second round of cloning, three recipients received an average of 105 SCNT embryos each and one went to term delivering four male piglets, one of which was a stillbirth. Birth weights of the three live clones were 0.76, 0.78, and 0.87 kg. Two of the clones died within 1 day of birth, and the remaining one survived to adulthood (Fig. 1D).
Microsatellite analysis
Of the 19 microsatellite markers tested, seven were found to be informative and could differentiate between donor cell lines as well as recipients. As shown in Table 2, the nuclear DNA genotypes indicated that the genotypes were identical between the cell lines and the SCNT clones derived from them, but differed from the surrogate recipient. This was the same for both first and second round SCNT clones. These data confirm that the piglets obtained were derived from the cell lines used for SCNT.
Table 2.
Microsatellite Analysis of Recipients, Cell Lines and Clones for Two Different Litters of SCNT Yucatans
| Microsatellite | Recipient 1 | Cell line YK1 | Clone YK1-1 | Clone YK1-2 | Recipient 2 | Cell line YK2 | Clone YK2-1 |
|---|---|---|---|---|---|---|---|
| SW632 | 186/188 | 194/194 | 194/194 | 194/194 | 180/186 | 178/178 | 178/178 |
| SW857 | 163/167 | 167/169 | 167/169 | 167/169 | 167/167 | 167/173 | 167/173 |
| S0143 | 175/184 | 175/175 | 175/175 | 175/175 | 181/181 | 175/184 | 175/184 |
| SO178 | 127/141 | 133/135 | 133/135 | 133/135 | 127/127 | 133/135 | 135/135 |
| SW980 | 133/141 | 133/143 | 133/143 | 133/143 | 133/146 | 141/141 | 141/141 |
| SW951 | 140/142 | 151/151 | 151/151 | 151/151 | 140/149 | 140/140 | 140/140 |
| S0090 | 259/263 | 261/265 | 261/265 | 261/265 | 259/263 | 265/265 | 265/265 |
Recipient 1 carried to term the first round clones. Recipient 2 carried to term the second round clones.
Mitochondrial D-loop analysis
In order to further confirm that the SCNT clones that were developed resulted from the SCNT procedure and not natural breeding, the mitochondrial D-loop region in all the animals and cell lines used in this experiment was haplotyped. The choice of region to analyze was based on previously identified Yucatan-specific haplotypes. As shown in Figure 2, two polymorphisms in the region sequenced were informative and capable of distinguishing between Yucatan-derived mitochondria and mitochondria derived from the occidental donor and recipients used for the SCNT. In one marker the Yucatan had a TCAT versus a CCAT for the occidental recipients. The second marker had a haplotype of TAACCC for the Yucatan versus a CGACCC or TGACCC for the occidental breeds. Figure 2 indicates that the recipients had the occidental-specific haplotype while the Yucatan cell lines had the Yucatan-specific haplotype. However, when the Yucatan clones and cell lines isolated from Yucatan clones were sequenced, it was confirmed that the mitochondria were derived from the occidental breed not from Yucatans.
FIG. 2.
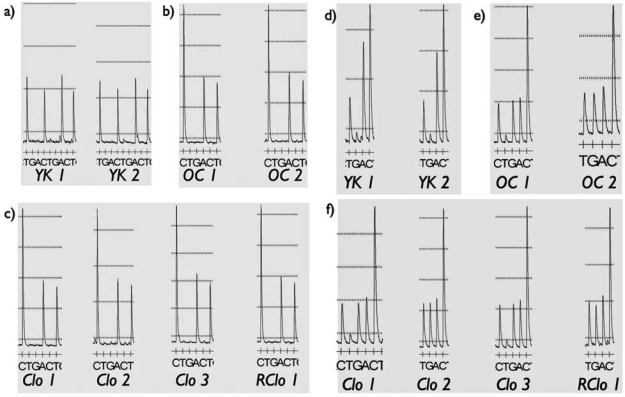
Mitochondrial D-loop sequences of polymorphic regions used to distinguish Yucatan from occidental/commercial swine mitochondria. Two polymorphisms were identified capable of differentiating among the different breeds. (A) D-loop sequence (TCAT) of Yucatan fetal cell lines (YK1 and YK2) used for SCNT. (B) Sequence (CCAT) of two recipients (OC1 and OC2) used to carry the SCNT clones to term. (C) Sequence of three first-round clones (Clo1, Clo2, Clo3) and one second-round clone (RClo 1). In all four cases the sequence matched the occidental/commercial genotype (CCAT), not the Yucatan one. (D) D-loop sequence (TAACCC) of Yucatan fetal cell lines (YK1 and YK2) used for SCNT. (E) Genotypes (CGACCC or TGACCC) of two recipients (OC1 and OC2) used to carry the SCNT clones to term. (F) Sequence of three first-round clones (Clo1, Clo2, and Clo 3) and one second-round clone (RClo 1). In all four cases the sequences matched the occidental/commercial genotypes (CGACCC or TGACCC) not the Yucatan one.
Phenotypic characteristics of Yucatan SCNT clones
As shown in Figure 3a and 3b, placental weights were significantly smaller (p < 0.018) in the Yucatan SCNT clones (n = 10) than the occidental SCNT clones (n = 24) (9.67 ± 2.12 versus 15.97 ± 1.4, respectively). Similarly, fetal weights of Yucatan SCNT clones (n = 10) where significantly smaller (p < 0.0005) than weights of occidental SCNT clones (n = 24) (0.79 ± 0.08 vs. 1.19 ± 0.05, respectively).
FIG. 3.
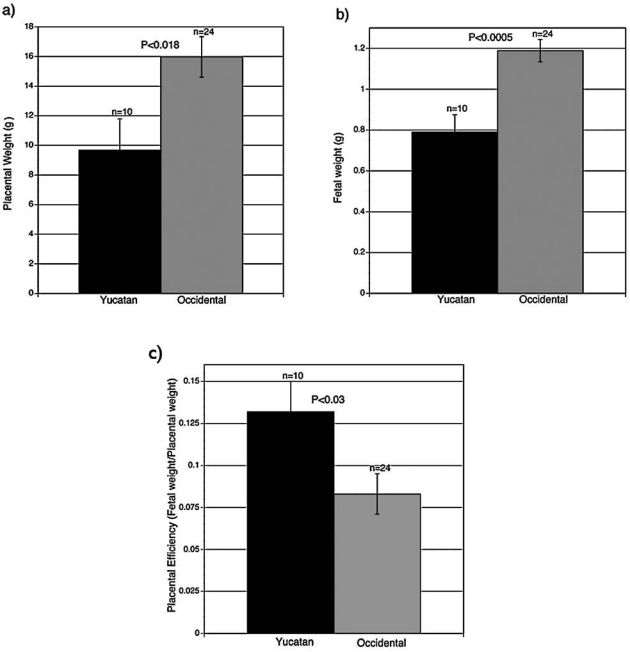
Fetal and placental weights of SCNT clones derived from either Yucatan cell lines or occidental/commercial origin cell lines. In both cases fetuses were collected at day 30 of gestation and placental and fetal weight recorded. n = number of observations. Yucatan clones had lower placental (p < 0.018) and fetal (p < 0.0005) weight that the control occidental/commercial origin SCNT clones.
Additionally, comparison of the postnatal mortality rate of the Yucatan clones to that previously reported by us using the same protocol and the same recipient herd (Estrada et al., 2007) indicates that the Yucatan clones have a higher rate of postnatal mortality. With respect to postnatal survival, three broad groups could be identified: clones in the upper weight range tended to survive to adulthood; clones in the bottom 25% percentile tended to die soon after birth (1 day or less); the remaining animals died within 3 days of birth unless they were provided additional feeding choices in the form of ad libidum milk replacement. In general, the piglets were highly active, and were aggressive in their suckling responses. However, the first 3 days postpartum were critical for long-term viability. Approximately half of the clones had difficulties during that initial period. At birth they were active and appeared healthy and strong, but behaved abnormally compared to control farm piglets. This abnormal behavior was characterized by periods of hyperactivity, which gradually decreased until the animals became nonresponsive and unable to suckle on their own. Manual feeding was attempted but the animals did not respond and died within 3 days of birth. Additionally, two of the males from one of the litters died soon after birth. They were normal and did not show any abnormal behavior. When necropsies were performed on these animals, no pathological abnormalities were found.
Discussion
The advent of somatic cell nuclear transfer combined with genetic manipulation of cells in culture has opened up a new venue for generation of complex genetically modified pigs for use in biomedical research (Kolber-Simonds et al., 2004; Lai et al., 2006; Ramsoondar et al., 2003; Takahagi et al., 2005). As this technology continues to mature, its impact on translational research will continue to increase, as swine are uniquely adapted to clinical development and testing of novel approaches. These can range from surgical interventions, gene, and cell-based therapies to pharmacological therapies. In each case the size and physiology of the swine provides unique advantages. However, with the expansion of the use of genetically modified swine has come the realization that for a more extensive utilization of this technology it is critical that breeds that are more amenable to biomedical applications are utilized. One such breed, the Yucatan minipig, has been used extensively for a range of biomedical applications. This breed is characterized by its small size and ease of use, yet it has not been used in genetic modification projects due to the costs associated with housing and maintaining a breeding colony of Yucatans. One option for overcoming this barrier is the utilization of more commonly available breeds as oocyte donors and recipients that can then be combined with Yucatan cell donors for generation of viable offspring that can be used as founder of transgenic Yucatan lines. We have now demonstrated that commercial occidental breeds of swine can be used in combination with Yucatan cell lines to produce both male and female SCNT offspring. Moreover, it was possible to obtain viable offspring after two rounds of SCNT cloning indicating that multiple rounds of transgenesis and recloning will be possible making the system even more useful. The microsatellite DNA analysis confirmed that the nuclear material in all of the SCNT clones was derived from the Yucatan's. Yet, the D-loop sequencing indicated that the mitochondrial DNA in the SCNT clones was of occidental origin. This not only demonstrates that the Yucatan we have generated are SCNT clones, but also that these clones are, in essence, nuclear-mitochondrial hybrids that differ from both the original Yucatan cell donors and the recipient oocytes, and that can only be produced using SCNT.
However, our data also indicates that the overall efficiency of SCNT, as measured by live piglets per SCNT embryos transferred, is lower that when we utilized occidental breed cells as nuclear donors (Estrada et al., 2007). With the Yucatan as nuclear donors we obtained less than 40% pregnancy rates to term, while with our occidental nuclear donors we obtain greater than 75% pregnancy to term. Moreover, Yucatan SCNT viable offspring are less than half those obtained with occidental nuclear donor embryos (average of five piglets per litter with occidental breeds versus less than two with the Yucatan). Additionally, when we collected two pregnancies to obtain fetal fibroblasts for a second round of SCNT, we observed a large number of resorbing fetuses in both recipients (four out of eight in one recipient, and 8 out of 12 in the second recipient). However, when we collected SCNT fetuses at similar stages of gestation in occidental breeds we observed only 10–20% resorbing fetuses. Thus, the Yucatan SCNT clones have considerably more fetal lethality at day 30 of gestation.
Also nonresorbing fetuses and some of their placentae were collected and weighed and both were found to be significantly lower in the Yucatan SCNT clones with the Yucatan fetuses being 34% smaller and the placentas 39% smaller than in the commercial/occidental SCNT concepti. Additionally, when ratios of fetal to placental weight, a measurement of placental efficiency, are calculated the Yucatan clones have a significantly higher ratio (Fig. 3c) that the occidental clones (0.13 ± 0.02 vs. 0.08 ± 0.01 for the Yucatan and occidental SCNT clones, respectively). Unfortunately, we do not have data from conventional Yucatan pregnancies at equivalent stages of gestation for comparison purposes. What we can state from these data is that both the fetuses, and in particular the placentas of the Yucatan SCNT clones, are smaller than occidental breed clones, and this may have contributed to the high fetal mortality in the Yucatan compared to SCNT clones from occidental breeds.
Additionally, the ability to survive postnatally is reduced in the Yucatan clones when compared to SCNT clones derived by us from commercial breeds (Estrada et al., 2007)). We believe this additional postnatal lethality is related to suckling difficulties associated with the small size of the piglets when compared with the relative large size of the nipple in the recipient gilts. This may have lead to hypoglycemia because of insufficient food taken during the first hours of life. If this is the case, simple management changes that include additional food availability should result in increased postnatal survival and further increase the value of this technology.
Overall, the reduced efficiencies seen in the Yucatan are unlikely to be an issue of technical variability or oocyte or recipient quality as we obtained higher efficiencies with occidental breeds using analogous oocyte sources as donors and the same recipient herd (Estrada et al., 2007). Additionally, the same individual generated both sets of observations. Thus, the lower viability of the Yucatan is caused by factors other than experimental variability. What those factors are remain to be determined, but could include mitochondrial incompatibility, or reduced compatibility between the Yucatan placenta and the occidental uterine environment.
Despite the reduced efficiencies, however, our data shows that it is possible to utilize commonly used commercial breeds as oocyte donors and recipients to generate Yucatan SCNT clones. The use of commonly available commercial breeds avoids the need for having access to Yucatan oocytes or synchronized Yucatan recipients, both of which would be prohibitively expensive for most laboratories. The successful cloning of Yucatan's using these procedures will greatly enhance the applicability of SCNT technology in biomedical research as many institutions are already experienced at working with the Yucatan minipig.
Acknowledgments
Authors are grateful to Dr. Larry Schook and Dr. Kefie for microsatellite analysis. Funding for the project was from grant HL51587 to J.P. and EY013978 to R.M.P.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Estrada J. Sommer J. Collins B., et al. Swine generated by somatic cell nuclear transfer have increased incidence of intrauterine growth restriction (IUGR) Cloning Stem Cells. 2007;9:229–236. doi: 10.1089/clo.2006.0079. [DOI] [PubMed] [Google Scholar]
- Eubanks D.L. Cooper R. Boring J.G. Surgical technique for long-term cecal cannulation in the Yucatan minipig (Sus scrofa domestica) J. Am. Assoc. Lab Anim. Sci. 2006;45:52–56. [PubMed] [Google Scholar]
- Hoshino Y. Uchida M. Shimatsu Y., et al. Developmental competence of somatic cell nuclear transfer embryos reconstructed from oocytes matured in vitro with follicle shells in miniature pig. Cloning Stem Cells. 2005;7:17–26. doi: 10.1089/clo.2005.7.17. [DOI] [PubMed] [Google Scholar]
- Kim K.I. Lee J.H. Li K., et al. Phylogenetic relationships of Asian and European pig breeds determined by mitochondrial DNA D-loop sequence polymorphism. Anim. Genet. 2002;33:19–25. doi: 10.1046/j.1365-2052.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- Kolber-Simonds D. Lai L. Watt S.R., et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L. Kang J.X. Li R., et al. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 2006;24:435–436. doi: 10.1038/nbt1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Lee S.H. Kim S., et al. Analysis of nuclear reprogramming in cloned miniature pig embryos by expression of Oct-4 and Oct-4 related genes. Biochem. Biophys. Res. Commun. 2006;348:1419–1428. doi: 10.1016/j.bbrc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Li R. Lai L. Wax D., et al. Cloned transgenic swine via in vitro production and cryopreservation. Biol. Reprod. 2006;75:226–230. doi: 10.1095/biolreprod.106.052514. [DOI] [PubMed] [Google Scholar]
- Mattern H.M. Lloyd P.G. Sturek M., et al. Gender and genetic differences in bladder smooth muscle PPAR mRNA in a porcine model of the metabolic syndrome. Mol. Cell Biochem. 2007;302:43–49. doi: 10.1007/s11010-007-9423-8. [DOI] [PubMed] [Google Scholar]
- Miyoshi K. Inoue S. Himaki T., et al. Birth of cloned miniature pigs derived from somatic cell nuclear transferred embryos activated by ultrasound treatment. Mol. Reprod. Dev. 2007;74:1568–1574. doi: 10.1002/mrd.20730. [DOI] [PubMed] [Google Scholar]
- Miyoshi K. Sato K. Yoshida M. In vitro development of cloned embryos derived from miniature pig somatic cells after activation by ultrasound stimulation. Cloning Stem Cells. 2006;8:159–165. doi: 10.1089/clo.2006.8.159. [DOI] [PubMed] [Google Scholar]
- Montezuma S.R. Loewenstein J. Scholz C., et al. Biocompatibility of materials implanted into the sub-retinal space of Yucatan pigs. Invest. Ophthalmol. Vis. Sci. 2006;47:3514–3522. doi: 10.1167/iovs.06-0106. [DOI] [PubMed] [Google Scholar]
- Pak Y. Stollberg-Zagar K. Mayersohn M. A porcine model for fixed drug eruptions in humans: the case of antipyrine in the Yucatan micropig. J. Appl. Toxicol. 2006;26:1–4. doi: 10.1002/jat.966. [DOI] [PubMed] [Google Scholar]
- Panepinto L.M. Phillips R.W. Wheeler L.R., et al. The Yucatan minature pig as a laboratory animal. Lab. Anim. Sci. 1978;28:308–313. [PubMed] [Google Scholar]
- Petters R.M. Wells K.D. Culture of pig embryos. J. Reprod. Fertil. 1993;48(Suppl.):61–73. [PubMed] [Google Scholar]
- Ramsoondar J.J. Machaty Z. Costa C., et al. Production of alpha 1,3-galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyl-transferase. Biol. Reprod. 2003;69:437–445. doi: 10.1095/biolreprod.102.014647. [DOI] [PubMed] [Google Scholar]
- Smith D.M. Martens G.W. Ho C.S., et al. DNA sequence based typing of swine leukocyte antigens in Yucatan miniature pigs. Xenotransplantation. 2005;12:481–488. doi: 10.1111/j.1399-3089.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- Svendsen O. The minipig in toxicology. Exp. Toxicol. Pathol. 2006;57:335–359. doi: 10.1016/j.etp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Swindle M.M. Thompson R.P. Carabello B.A., et al. Heritable ventricular septal defect in Yucatan miniature swine. Lab. Anim. Sci. 1990;40:155–161. [PubMed] [Google Scholar]
- Takahagi Y. Fujimura T. Miyagawa S., et al. Production of alpha 1,3-galactosyltransferase gene knockout pigs expressing both human decay-accelerating factor and N-acetylglucosaminyltransferase III. Mol. Reprod. Dev. 2005;71:331–338. doi: 10.1002/mrd.20305. [DOI] [PubMed] [Google Scholar]
- Walker S.C. Shin T. Zaunbrecher G.M., et al. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells. 2002;4:105–112. doi: 10.1089/153623002320253283. [DOI] [PubMed] [Google Scholar]
- Witczak C.A. Wamhoff B.R. Sturek M. Exercise training prevents Ca2+ dysregulation in coronary smooth muscle from diabetic dyslipidemic yucatan swine. J. Appl. Physiol. 2006;101:752–762. doi: 10.1152/japplphysiol.00235.2006. [DOI] [PubMed] [Google Scholar]


