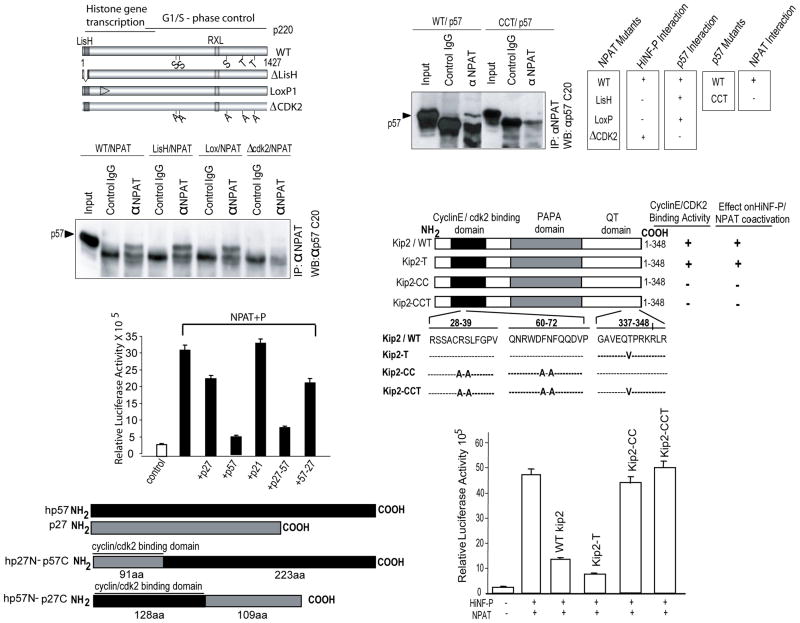
Fig. 6.
p57KIP2 and p220NPAT form specific complexes. (A) Wild type p220NPAT forms a complex with p57KIP2 via residues involved in CDK2 phosphorylation. Immuno-complexes were obtained from Cos7 cells transfected with expression vectors for p57KIP2 (25 ng/well) and wild type or mutant p220NPAT proteins (200 ng/well). Whole cell protein (~100 μg) was precipitated with anti-p220NPAT antibody (1 μg) antibody, and analyzed by western blotting using an anti-rabbit-p57KIP2 antibody (1:3,000 dilution; secondary goat anti-rabbit IgG antibody= 1:5,000 dilution). Wild type p220NPAT and two p220NPAT mutants (LisH, deletion of aa 3-38; LoxP1: alanine substitutions between aa 121-145, respectively) interact with p57KIP2, but the p220NPAT -ΔCDK2 mutant with alanine substitution in five C-terminal CDK2 phosphorylation sites (S/T) does not. (B) The N-terminal cyclin binding of wild type p57KIP2 supports interactions with p220NPAT. Wild type p57KIP2 and a p57KIP2 mutant with amino acid substitutions in the cyclin binding domain (see C) were expressed in Cos7 cells, and immunoprecipitates were obtained as described above (see A). Complexes with p220NPAT are only formed with wild type p57KIP2 but not with p57KIP2-CCT as indicated. The immunoprecipitation results presented here were correlated with those obtained for HiNF-P and p220NPAT previously (lower panel). (C) Inhibition of H4 gene transcription requires the cyclin binding domain of p57KIP2. Co-activation assays for p220NPAT/HiNF-P were performed with cells co-transfected with vectors expressing wild type or mutant p57KIP2 (i.e., T, CC and CCT; 25 ng/well) and luciferase activity for each mutant is plotted. (D) As in Panel C, but using reciprocal in the C-termini of p57KIP2 and p27KIP1 are swapped.