Figure 2.
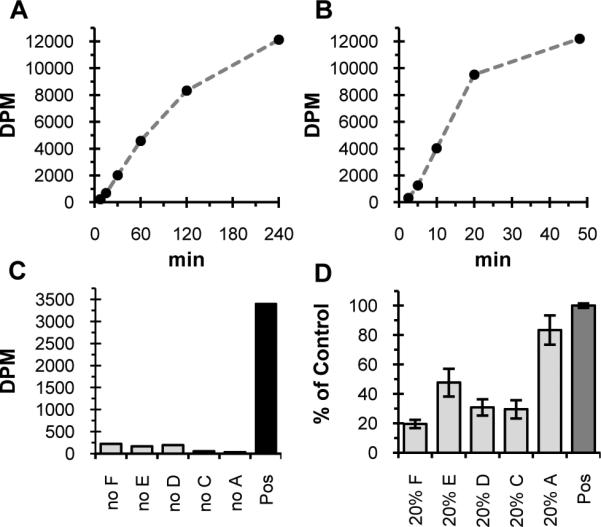
Transfer of radioactivity to an organic extract in the Pgl pathway screen (27000 DPM = theoretical yield). A) Initial activity of PglF, PglE, PglD, PglC, PglA assay with UndP measured with the lipophilicity-based assay (0.04% Triton X-100, 42 nM PglF, 146 μM PglE, 236 nM PglD, 52.5 nM PglC, 444 nM PglA). B) Activity of PglF, PglE, PglD, PglC, PglA, DGK assay with UndOH (0.04% Triton X-100, 84 nM PglF, 700 nM PglE, 675 nM PglD, 105 nM PglC, 222 nM PglA, 272 nM DGK). C) Transfer of radioactivity to organic phase is dependent on each enzyme. Radioactivity transferred to the organic phase at 30 min by PglF, PglE, PglD, PglC, PglA assay with UndP (control) compared to when individual enzymes are removed (0.04% Triton X-100, 42 nM PglF, 146 nM PglE, 1.2 nM PglD, 53 nM PglC, 444 nM PglA). Similar results were observed when UndOH and DGK used instead of UndP (not show). D) Assay is optimized for maximum sensitivity to inhibition of PglF, PglE, PglD, and PglC. Lipophilic radioactivity when each enzyme is individually lowered five-fold from control ([Triton X-100], [enzyme] as in C). Data normalized from three separate runs where lipophilic radioactivity in control was 2000 to 2500 DPM. Error bars represent the standard error of the mean (n = 3 for 20% enzyme data, n = 9 for control).
