Table 2.
Carbonate Opening Experiments
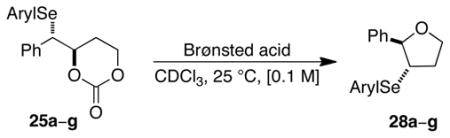 | |||||||
|---|---|---|---|---|---|---|---|
| entry | Aryl | carbonate | acid, equiv | ether | conv.,a% | e.r.b | e.s. (%) |
| 1c | 4-MeOC6H4 | 25b | TFA, 0.1 | 28b | 46 | 52.5:47.5 | 5 |
| 2c | Ph | 25a | TFA, 0.1 | 28a | 17 | 57.4:42.6 | 17 |
| 3c | 2,4,6-(i-Pr)3C6H2 | 25c | TFA, 0.1 | 28c | 48 | 62.8:37.2 | 30 |
| 4c | 4-CF3C6H4 | 25d | TFA, 1.0 | 28d | 100 | 61.8:38.2 | 27 |
| 5c | 2-CF3C6H4 | 25e | TFA, 1.0 | 28e | 100 | 67.2:32.8 | 40 |
| 6c | 4-NO2C6H4 | 25f | TFA, 1.0 | 28f | 54 | 93.6:6.4 | >99 |
| 7d | 2,6-(CF3)2C6H3 | 25g | MsOH, 1.0 | 28g | 94 | 93.7:6.3 | >99 |
Conversion determined by monitoring reaction progress by 1H NMR spectroscopy.
Determined by CSP-SFC analysis.
Reaction time was 24 h.
Reaction time was 2 h.
