Table 5.
Substrate Scope for Catalytic Asymmetric Selenoetherification
| entry | substrate | producta | yield,b % | e.r.d |
|---|---|---|---|---|
| 1. |
(E)-29 |
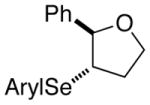 28f |
97% | 68.9:31.1 |
| 2. |
(E)-30 |
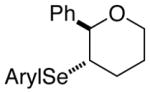 31 |
86% | 74.9:25.1 |
| 3. |
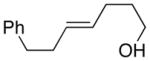 (E)-36 |
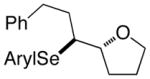 37 |
94% 37:38 = 1.4:1c |
78.8:21.2 |
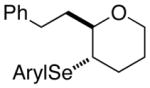 38 |
85.1:14.9 | |||
| 4. |
(E)-39 |
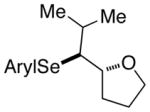 40 |
86% 40:41 = 4.5:1c |
82.6:17.4 |
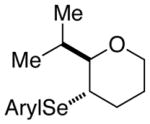 41 |
83.8:16.2 | |||
| 5. |
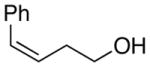 (Z)-29 |
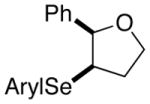 42 |
80% | 50.9:49.1 |
Aryl = 2-nitrophenyl.
Reaction conditions: alkene (1.0 mmol), 34 (1.1 mmol), MsOH (1.0 equiv), 35m (0.1 mmol), CHCl3 (0.1 M), 25 °C.
Exo:Endo ratios determined by 1H NMR spectroscopic analysis of the crude reaction mixture.
Determined by CSP-SFC analysis
