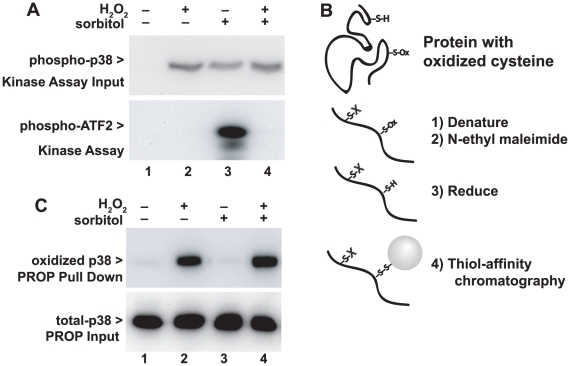
Figure 1. Thiol oxidation and inhibition of p38.
Panel A. Hydrogen peroxide inhibits p38 kinase activity but not phosphorylation. Thirty minutes before harvest, HeLa cells grown in 6-well plates were treated with 10 mM H2O2. Ten minutes later, some wells were treated with 0.4 M sorbitol to activate p38 through osmotic shock. (top) Both H2O2 and sorbitol induced phosphorylation of p38. (bottom) P38 activity measured with an in vitro kinase activity was only present in osmotically-stimulated cells not treated with hydrogen peroxide. Kinase activity was not correlated with phosphorylation of p38. The figure is representative of five independent experiments. Panel B. Outline of the PROP procedure to purify reversibly thiol-oxidized proteins (see details in Methods). Cultured cells are rapid fixed in TCA, then proteins denatured and solubilized in guanidine buffer containing NEM to block non-oxidized thiol groups on proteins. Oxidized proteins are reduced with DTT, then proteins with newly revealed free thiols isolated using thiol-affinity chromatography. Proteins are released from the affinity beads and subjected to SDS-PAGE and immunoblotting, compared to an aliquot of total proteins taken at step 1. Panel C. Hydrogen peroxide treatment results in reversible thiol oxidation of p38 detected using PROP. HeLa cells treated as in Panel A were subjected to PROP. (top) Reversible oxidation of p38 was detected in cells treated with 10 mM H2O2 but not in untreated cells. (bottom) Treatments did not alter the abundance of p38 in total lysates. The figure is representative of over 20 experiments.