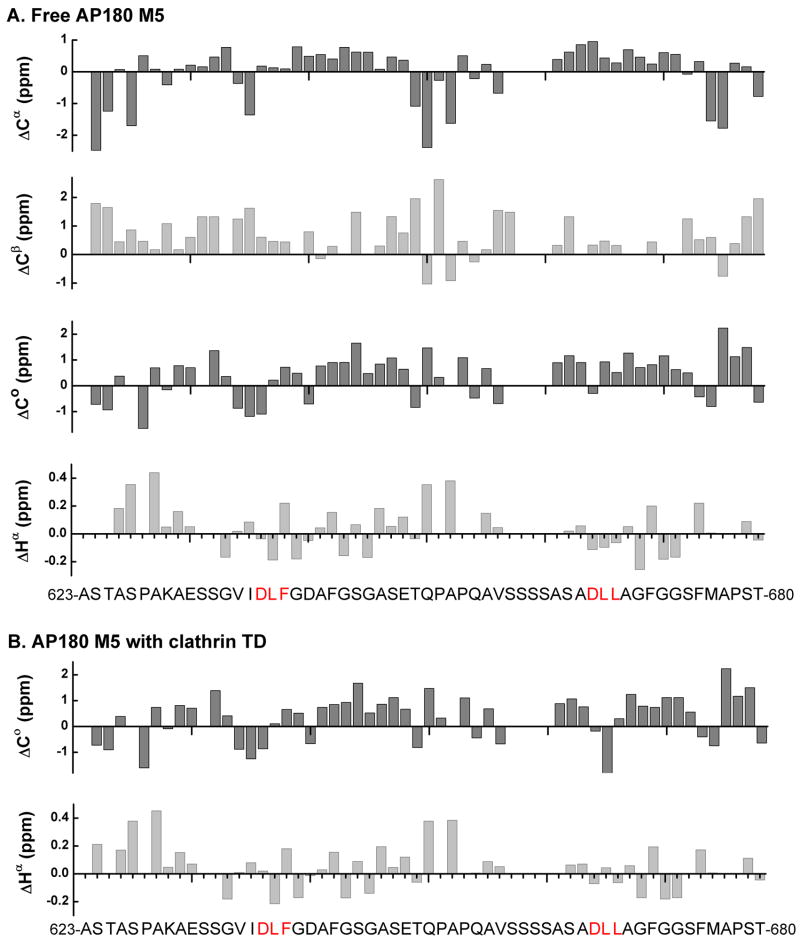
Figure 6.
Analysis of secondary chemical shifts indicates that AP180 M5 contains no regions of α helical or β street structure, in either the free or bound state. It has been reported that for residues in stable α helices, the average secondary chemical shifts are 2.5 ppm for 13Cα and −0.38 ppm for 1Hα, with near random coil values for 13Cβ and positive values for 13Co. In stable β sheets, the average secondary chemical shifts are −2.0 ppm for 13Cα, 2.5 ppm for 13Cβ and 0.38 ppm for 1Hα, with negative values for 13Co75. A. Secondary chemical shifts of free 500 μM 15N-13C labeled AP180 M5 for 13Cα, 13Cβ, 13Co and 1Hα were calculated by subtracting random coil shifts corrected for sequence-dependent variations from the experimental chemical shifts. No regions with patterns indicative of either α helix or β sheet were identified. B. Secondary chemical shifts of 500 μM 15N-13C labeled AP180 M5 with 500 μM unlabeled clathrin TD for 13Co and 1Hα were calculated in the same way. No regions with patterns indicative of either α helix or β sheet were identified.