Abstract
Microglial hyperactivity contributes to neuronal damage resulting from CNS injury and disease. Therefore, a better understanding of endogenous microglial receptor systems that can be exploited to modulate their inflammatory functions is important if better, neuroprotective therapeutics are to be designed. Previous studies from our lab and others have demonstrated that the P2X7 purinergic receptor agonist BzATP attenuates microglial inflammatory mediator production stimulated by lipopolysaccharide (LPS), suggesting that purinergic receptors may be one such receptor system that can be used for manipulating microglial activation. However, although P2X7 receptor activation is well recognized to regulate processing and release of cytokines, little is known concerning its role in regulating the transcription of inflammatory genes, nor the molecular mechanisms underlying these transcriptional effects. In the present studies, we identify that the transcription factors early growth response (Egr)-1, -2 and -3 are downstream signaling targets of P2X7 receptors in microglia, and that their activation is sensitive to MEK and p38 mitogen-activated protein kinase (MAPK) inhibitors. Moreover, using RNAi, we demonstrate that Egr factors and P2X7 receptors are necessary for BzATP-mediated attenuation of iNOS, and stimulation of TNF-α and IL-6 gene expression. BzATP also attenuates neuronal death induced by LPS conditioned medium, and P2X7 receptors are required for this effect. These studies are the first to identify Egr factors as regulators of inflammatory gene expression following P2X7 receptor activation, and suggest that P2X7 receptors may utilize the MAPK-Egr pathway to exert differential effects on microglial inflammatory activities which are beneficial to neuron survival.
Introduction
Many immune properties of microglia, CNS-resident, phagocytic immune cells, are controlled by P2 purinergic receptors, for which adenine nucleotides are the endogenous ligands. Whereas the actions of the P2X7 receptor in particular have been assigned to increased microglial processing and release of mature cytokines including interleukin (IL)-1α, IL-1β and IL-18 (Ferrari et al. 1996; Perregaux et al. 2000), as well as the release of other cytokines and inflammatory mediators including tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), plasminogen and matrix metalloproteinase-9 (Boucsein et al. 2003; Brautigam et al. 2005; Gu and Wiley 2006; Hide et al. 2000; Inoue et al. 1998), the molecular mechanisms underlying potential stimulatory or inhibitory transcriptional effects of P2X7 receptors on the expression of these or other inflammatory mediators have not been well characterized. Activation of the transcription factors NF-κB and NFAT by P2X7 receptors in microglia have long been known (Ferrari et al. 1999; Ferrari et al. 1997), but surprisingly, the gene targets of these transcription factors in response to P2X7 receptor activation in microglia have not been identified. However, in this regard, NFAT was very recently shown to mediate the transcriptional effects of P2X7 receptors on CC-chemokine ligand (CCL)3 (also called macrophage inflammatory protein (MIP) -1 alpha) expression in microglia (Kataoka et al. 2009), which is the first report to directly link these receptors to a transcription factor necessary for subsequent inflammatory gene expression in any cell type.
Work from our laboratory and others’ has pointed to a role for P2 purinergic receptors in reducing microglial production of inflammatory mediators stimulated by gram-negative bacterial lipopolysaccharide (LPS) (Boucsein et al. 2003; Brautigam et al. 2005; Ogata et al. 2003). Although all purinergic receptors involved in these effects have not yet been elucidated, the P2X receptor agonist BzATP decreases the expression of several LPS-stimulated inflammatory mediators (Boucsein et al. 2003; Brautigam et al. 2005) including that of iNOS. Because BzATP is an agonist of several P2X receptor subtypes (Burnstock and Knight 2004), and the mechanisms underlying the inhibitory effects of BzATP on microglial gene transcription are not known, the first hypothesis we tested in the present studies was that P2X7 receptors in specific, mediate the inhibitory effects of BzATP on LPS-stimulated iNOS gene expression in microglia.
P2X7 receptors are well-known to promote the activation of the mitogen-activated protein (MAP) kinases ERK-1/-2 and p38 in both microglia and macrophages (reviewed in (Potucek et al. 2006; Watters et al. 2001)), although alone, activation of these pathways is not sufficient to promote iNOS expression, for example (Aga et al. 2004; Brautigam et al. 2005). MAP kinases are requisite for controlling inflammatory gene expression in many cell types (Aga et al. 2004; Bhat et al. 1998; Watters et al. 2002) via their activation of transcription factors critical for inflammatory gene expression (McCubrey et al. 2000; Watters et al. 2001; Williams et al. 2008). Because MAP kinases can regulate many transcription factors necessary for inflammatory gene expression, we performed a microarray analysis to narrow down potential BzATP-induced transcription factor targets. We found an up-regulation of early growth response (Egr) target genes in microglia treated with BzATP for 4 hours (JW and PJB unpublished data), suggesting that Egr transcription factors may mediate some of the effects of BzATP in microglia. The four known members of this immediate early gene family: Egr-1 (also known as NGFI-A, Krox-24 or Zif268), Egr-2/KROX-20, Egr-3 and Egr-4/NGFI-C are regulated by MAP kinases (Hipskind et al. 1994; McCubrey et al. 2000), and their transcriptional activities control the expression of many inflammatory genes including IL-6 and tumor necrosis factor (TNF)-α (Decker et al. 2003; Prince et al. 2007). Consequently, in microglia, we next tested the hypotheses that: 1) MAP kinase activation mediates Egr transcription factor induction in response to BzATP treatment, and 2) that Egr factors are necessary for controlling inflammatory gene expression following BzATP treatment.
Materials and Methods
Materials
LPS (E.coli 0111:B4), 3’-O-(4-benzoylbenzoyl)-adenosine 5’-triphosphate (BzATP), adenosine 5’-triphosphate (ATP), alpha,beta methylene-ATP (α,β Me-ATP), adenosine 5’-triphosphate-2’,3’-dialdehyde (oATP), and brilliant blue G (BBG) were purchased from Sigma Chemical Company (St. Louis, MO). 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene (U0126), 4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole (SB202190), 1-[N, O-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]- 4-phenylpiperazine (KN-62), 1,4-diamino-2,3-dicyano1,4-bis(methylthio)butadiene (U0124) were obtained from EMD Biosciences/Calbiochem (San Diego, CA). 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole (SB203580), 2’-Amino-3’-methoxyflavone (PD98059) were purchased from the Promega Corporation (Madison, WI). A-438079 was purchased from Tocris Bioscience (Ellisville, MO). YO-PRO-1 and propidium iodide were purchased from Molecular Probes/Invitrogen (Carlsbad, CA).
Cell Culture
Murine N9 microglial cells, kindly provided by Dr. Paula Ricciardi Castagnoli (University of Milan) (Righi et al. 1989), were routinely cultured in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Herndon, VA) supplemented with 5% bovine growth serum (Hyclone, Logan, UT) and 100 U/ml penicillin/streptomycin (Cellgro) in 100 mm Sarstedt plates. Cells were grown to ~90% confluency and passaged every 2 days. For experimentation, cells were plated at densities of 4×105 per well in 12-well plates or 2×105 per well in 24 well plates. The next day, the cells were treated in triplicate as specified in the figures. For nucleotide treatments, concentrations above 150 μM for BzATP, and 300 μM for ATP were not used because they promote significant cell death (data not shown and (Brautigam et al. 2005)). Murine hippocampal HT22 neurons (kindly provided by Dr. Daniel Dorsa, Oregon Health and Sciences University, Portland, OR) were routinely cultured as described for N9 microglia above, except they were grown in DMEM with 5% FBS. For experimentation, HT22 cells were plated at 1×104 cells/well in 96 well plates.
Primary microglia shaken from mixed glial cultures were obtained from neonatal ICR-CD1 mice (~postnatal days 3-5) and cultured in DMEM supplemented with 10% fetal bovine serum (Hyclone) and 100 U/ml penicillin/streptomycin (Cellgro) as previously described (Nikodemova et al. 2006). For experimentation, primary microglia were plated at 105 cells/well in 96 well plates for NO assays, or 2×105 cells/well in a 24 well plate for gene expression analysis.
HT22 neuronal cell death
HT22 hippocampal neurons were plated in 96 well plates (1×104 cells/well) and treated in triplicate with conditioned medium from microglia that were treated overnight with vehicle (250 mM Hepes), LPS (1 μg/mL), BzATP (150 μM) or both LPS and BzATP together for 18-22 hours. Microglial conditioned medium was added to HT22 growth medium to obtain a final ratio of 1:1. Neuronal viability was evaluated using the CellTiter96 Aqueous Non-radioactive Cell Proliferation Assay (Promega, Madison, WI), according to the manufacturer's instructions. This proliferation assay assesses metabolic/mitochondrial function by measuring the conversion of a tetrazolium compound (MTS) to a soluble formazan product that can be quantified spectrophotometrically. The data shown are representative of 4-6 independent experiments.
YO-PRO dye uptake assay
Parental N9, shP2X7/N9, shP2X4/N9 and shEgr/N9 microglia (5×105 cells) were washed in HEPES-buffered saline (HBS: 130 mM NaCl, 5 mM KCl, 0.5 mM CaCl2, 20 mM HEPES (pH 7.4), 0.1% BSA and10 mM glucose), and then resuspended in fresh HBS. The dye uptake reaction was initiated by addition of YO-PRO-1 (1-2 μM final concentration) with and without BzATP (300 μM). Cells were incubated at room temperature for 15 min, and then washed and resuspended in fresh HBS containing propidium iodide (5 μg/ml). Flow cytometry and data analysis with the exclusion of dead cells was then performed as described previously (Aga et al. 2002). The data shown are representative of at least 3 independent experiments.
Measurement of NO Production
Microglia were plated at a density of 2×105 per well in 24-well plates and treated for 24 hours. Cells under these conditions were stimulated with LPS (0.1-1 μg/mL) in the presence or absence of BzATP (150 μM) as indicated in the figures. In experiments using all P2 receptor antagonists except oATP, cells were pretreated with the antagonists for 15 or 30 minutes prior to stimulation with LPS and/or BzATP; oATP pretreatment was 1 hr. For NO determination, the medium was removed and analyzed for the concentration of nitrite (a stable break down product of nitric oxide generation) using the Greiss reagent (Mitchell et al. 1992).
Ras activation
Microglia were treated with vehicle (250 mM Hepes) or BzATP (250 μM) for 5 minutes and cells were lysed in lysis buffer (10% glycerol, 50 mM Tris-HCl: pH 7.4, 200 mM NaCl, 1% NP-40 and 2 mM MgCl2). The cell lysates were then pre-cleared using glutathione-agarose beads (Pierce) and incubated at 4°C for 10 minutes, followed by centrifugation to remove the beads. The pre-cleared supernatants were incubated with glutathione-agarose beads conjugated to a glutathione-S-transferase (GST) fusion protein of the Ras-binding domain of Raf (Upstate Biotechnology) for 30 minutes at 4°C. The beads were washed 3 times with lysis buffer and active Ras proteins were eluted with sample buffer, followed by boiling for 5 minutes. The proteins were separated by SDS-PAGE, and processed for immunoblot analysis using anti-Ras antibodies (Transduction Laboratories; Lexington, KY). Data shown are representative of at least 2 independent experiments.
Immunoblot Analyses
Western blots using whole cell lysates were performed as we have described previously (Brautigam et al. 2005). The levels of p44/42, p38, iNOS and P2X7 were ascertained using: anti-active p44/42 antibodies (1:1000, Cell Signaling, Danver, MA), anti-active p38 MAP kinase antibodies (1:1000, Cell Signaling, Danver, MA), anti-iNOS antibodies (1:2000; Transduction Laboratories, Lexington, KY), and anti-P2X7 antibodies (1:200; Alomone Labs, Jerusalem, Israel). The immunoreactive bands were visualized using secondary antibodies conjugated to horseradish peroxidase (Santa Cruz) and chemiluminescent detection methods (Pierce Biochemical Company). To confirm protein loading, membranes were probed with antibodies recognizing GAPDH, Grb-2 or β-actin (1:5000; Santa Cruz). The immunoblot data shown are representative of at least 3 independent experiments.
Real time PCR
Murine N9 microglia were grown in 6 well plates as detailed above. Cells were lysed in Tri- Reagent (Sigma), and total RNA was harvested according to the manufacturer's protocol. Reverse transcription PCR (RT-PCR) was performed using 1μg of total RNA as a template for the reverse transcription reaction using random hexamers and ImProm-II Reverse Transcriptase (Promega) according to the manufacturer's protocol. Quantitative RT-PCR was performed by monitoring in real-time the increase in fluorescence of the SYBR-GREEN dye using the TaqMan 7300 Sequence Detection System (Applied Biosystems). The relative amounts of each gene were determined using the comparative CT method. CT values were normalized to the relative levels of β-actin in each sample. Primer sequences for the following murine genes were used: β-actin Forward-AGGATGCCACGGCTGATG and Reverse-ACAACAGGAGGTCGTACCAGA; BDNF Forward-CCCATCACAATCTCACGGTATTC and Reverse- TGCGGAGGGTCTCCTATGAA; Egr 1 Forward-CATGCCTGGCCCTTGCT and Reverse-GAGGGCCCAAACCCATTTT; Egr 2 Forward-GGTTGGGAGTTGCTGATTCCT and Reverse-ATTAAAGTGCCACATCAGTTTTGCT; Egr 3 Forward-ATTCCCCCCAGGATTACCAA and Reverse-GTGGTACAGGTTGTAGTCAGGAATCA; IL-6 Forward-GTGGCTAAGGACCAAGACCA a n d Reverse-GGTTTGCCGAGTAGACCTCA; IL-10 Forward-AAGCTGAAGACCCTCTGGATACA and Reverse-CCACTGCCTTGCTTTTATTCTCA; iNOS Forward-CATCAGGTCGGCCATCACTG and Reverse-CGTACCGGATGAGCTGTGAATT; NAB-1 Forward-CTGGCCAGGCAGGTTTCTC and Reverse-TGGCACAGATTCCTGGAAGTC; NAB-2 Forward-GGAGAGGATCTTCCGGAGTTTC and Reverse-TCCGCGCCAGCTTCTTATT; P2X7 Forward-CCACAACTACACCACGAGAAAC and Reverse-ACTTCTTGGCCCTTGACATCTT; TGFβ Forward-TGACGTCACTGGAGTTGTACGG and Reverse-GGTTCATGTCATGGATGGTGC; TNF-α Forward-TGCCACTTCATACCAGGAGA and Reverse-CCGGACTCCGTGATGTCTA; VEGF Forward-TTGAGACCCTGGACATCT and Reverse-CACACAGGACGGTTGAAGA.
RNA interference
N9 microglia plated in 96 well plates (3×104 cells/well) were mock infected, or infected with Sigma MISSION™ lentivirus particles (1.2×104 TU/well) encoding short hairpins to the murine P2X7 or P2X4 receptors, or Egr-1, Egr-2 and Egr-3 factors in the presence of hexadimethrine bromide (final concentration 8 μg/mL). Catalog numbers for Sigma lentiviral particles with which we made stable cell populations are as follows: SHVRSC-TRCN00000: 68568 (P2X7); 68534 (P2X4); 81626 (Egr-1); 81678 (Egr-2); and 96140 (Egr-3). All vectors contained puromycin resistance to facilitate stable cell clone selection. The medium was changed the day after infection, and cells were allowed to grow to confluence at which time cell populations containing stably integrated short hairpins were selected with puromycin (10 μg/mL) over several passages; antibiotic concentration was gradually decreased to 2 μg/mL, the final dose used for routine stable cell line maintenance. Because of functional Egr compensation, we had to knock down all three Egr factors expressed in our cells in order to assess their role in P2X7 signaling (data not shown). Cells stably expressing short hairpins to the P2X7 receptor (shP2X7/N9), P2X4 receptor (shP2X4/N9) or to the combination of Egr-1, -2 and -3 factors (shEgr/N9) were then plated for experiments as indicated above.
Statistical Analysis
Statistical analyses were performed using an unpaired t-test or one-way ANOVA pre hoc test and the Tukey-Kramer Multiple Comparisons, Dunnett Multiple Comparisons, or Fisher LSD post hoc tests. Statistical significance was set at p < 0.05. Quantitative data are expressed as the mean ± SEM of n ≥ 3 independent measurements.
Results
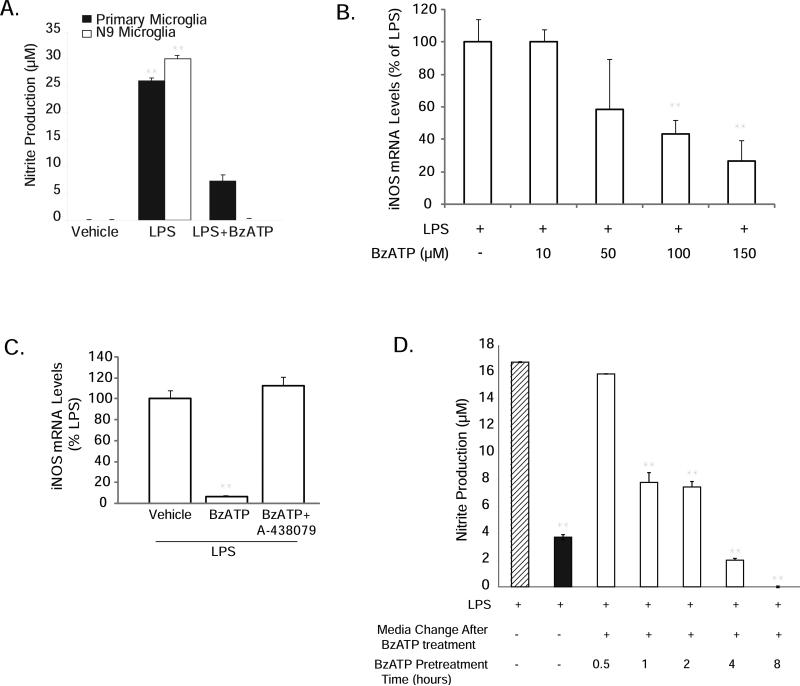
BzATP acts on P2X7 receptors to decrease LPS-stimulated inducible nitric oxide synthase (iNOS) and nitric oxide (NO) levels in a time frame consistent with the need for immediate early gene transcription
The P2X receptor agonist BzATP decreases LPS-stimulated NO production both in primary microglia and in the N9 microglial cell line (Fig. 1A), consistent with our previous report in BV-2 microglia (Brautigam et al. 2005). BzATP dose-dependently reduces LPS-induced iNOS mRNA levels (Fig. 1B), and this attenuation by BzATP was completely blocked by the new and highly selective P2X7 receptor antagonist A-438079 (Fig. 1C). These effects on iNOS expression were also observed at the protein level where the P2X7 receptor antagonists BBG (Supplementary Fig. 1) and KN-62 (data not shown) prevented BzATP from reducing LPS-stimulated iNOS protein levels. To begin to investigate the signaling mechanisms involved in these effects, we performed time courses of BzATP exposure and washout, to determine the length of time required for BzATP to inhibit LPS-stimulated NO production (Fig. 1D). Microglia were treated with BzATP for 0.5 hours to 8 hours as indicated in the figure, after which time the nucleotides were removed by medium washout (white bars). The cells were then stimulated overnight with LPS in fresh medium (striped bar), and nitrite accumulation in the tissue culture medium was analyzed using the Griess reagent (Mitchell et al. 1992). As a control, BzATP was also added concomitantly with LPS with no medium wash out (black bar). BzATP statistically significantly reduced NO production within 1 to 2 hours, a time course consistent with the synthesis of immediate early gene transcription or translation. Due to their inability to be washed away and their subsequent interference with LPS signaling, we were unable to use standard transcription or translation inhibitors to directly test this idea (data not shown). However, as discussed above, our microarray data suggested that Egr immediate early genes might be reasonable candidates for transcription factors whose expression could be induced by BzATP within the 1 to 2 hour time window. Importantly, we identified multiple, putative cis-acting elements for Egr factors in the 5’ flanking region of the iNOS gene (Table 1), using the Genomatix program MatInspector.
Figure 1. BzATP-induced attenuation of LPS-induced iNOS/NO production in murine microglia occurs within 2 hours and is sensitive to P2X7 antagonists.
(A) BzATP treatment attenuates LPS-stimulated NO production. N9 and primary murine microglia were treated with vehicle (250 mM Hepes), LPS (100 ng/mL) or co-treated with LPS+ BzATP (150 μM) for 16-20 hours. Nitrite levels were measured in the medium the following day. **p< 0.01 vs. vehicle treatment. (B) LPS-induced iNOS mRNA levels are dose-dependently reduced by BzATP treatment. The data are graphed as percent expression relative to LPS treatment. **p<0.01 vs. LPS treatment. (C) LPS-induced iNOS mRNA levels are reduced by BzATP, an effect that is reversed by the novel P2X7 antagonist A-438079. **p<0.01 vs. LPS treatment. (D) BzATP reduces LPS-stimulated NO production within 1-2 hours after treatment. Cells were treated with LPS alone (hatched bar), co-treated with LPS+BzATP (black bar), or pre-treated with BzATP for times indicated (3rd through 7th bars, respectively), prior to medium washout and stimulation with LPS. All treatments were performed in triplicate and the means ± SEM for the data shown are representative of 4-7 independent experiments. **p<0.01 vs. LPS treatment alone.
Table 1. Putative Egr Binding Sites in 5' Flanking Region of iNOS Gene.
Using the Genomatix program MatInspector, we identified four putative Egr factor binding sites in the first 4.182kb of the 5’ flanking region of the mouse iNOS gene. Capital letters indicate the core consensus used by the MatInspector algorithm, and underlined nucleotides represent residues which are highly conserved. Sites 1 and 4 had 100% similarity to the core sequence in the algorithm, whereas sites 2 and 3 had 77% and 76%, respectively. There is considerable heterogeneity among Egr factors for the nucleotide sequences to which they bind, so specific Egr factors cannot be assigned to particular Egr binding sites using solely an informatics approach.
| Site | Position From Transcription Start Site (+1) | Nucleotide Sequence |
|---|---|---|
| 1 | -379-395 | ctctgcggGGGCtgaat |
| 2 | -2787-2803 | gcatGGGTtggtgagat |
| 3 | -3358-3374 | gtatGTGTgggtgactt |
| 4 | -3758-3774 | actaGGGAgggaggctg |
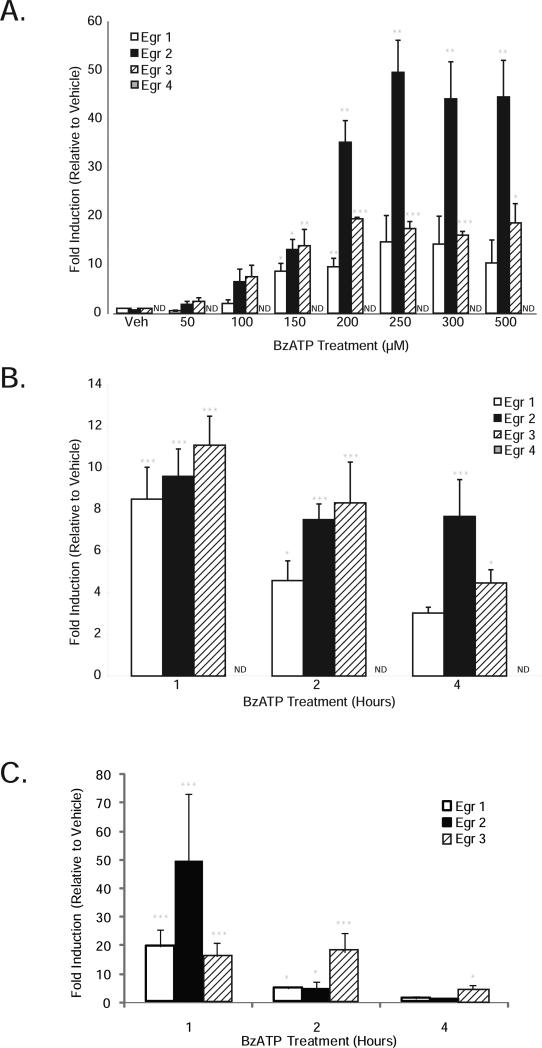
BzATP induces Egr expression in a time and dose-dependent manner
We next tested the ability of BzATP to promote Egr factor induction. Treatment of microglia with BzATP for 1 hour dose-dependently increased Egr-1, Egr-2 and Egr-3 mRNA levels; Egr-4 mRNA levels were very low and often not detectable (ND; Fig. 2A). A time course of BzATP treatment demonstrated peak Egr expression at 1 hour in both N9 microglia (Fig. 2B) and primary microglia (Fig. 2C), consistent with the time course of NO inhibition (Fig.1D), previous reports of BzATP treatment of adventitial fibroblasts (Gerasimovskaya et al. 2002) and HEK 293 cells transiently transfected with P2X7 receptors (Stefano et al. 2007). Egr-4 expression in N9 microglia did not change following BzATP treatment at any time point we assessed up to 24 hours, so it was not evaluated further. Because 150μM BzATP was the lowest dose that gave statistically significant Egr stimulation, all subsequent Egr studies were performed using this concentration.
Figure 2. BzATP dose-dependently stimulates Egr expression which is maximal at 1 hour.
Total RNA was isolated from N9 (A, B) and primary (C) microglia treated with vehicle (250 mM Hepes) or BzATP (doses and times as indicated in the figure), and qRT-PCR was performed to assess expression levels of the transcription factors Egr-1 (white bars), Egr-2 (black bars), and Egr-3 (striped bars). Egr-4 levels were very low and often not detectable (ND). The data are graphed as fold induction relative to vehicle treatment for each gene. Data represent the mean of at least 3 independent experiments ± SEM. * p<0.05, ** p<0.01 and *** p<0.001 vs. vehicle treatment.
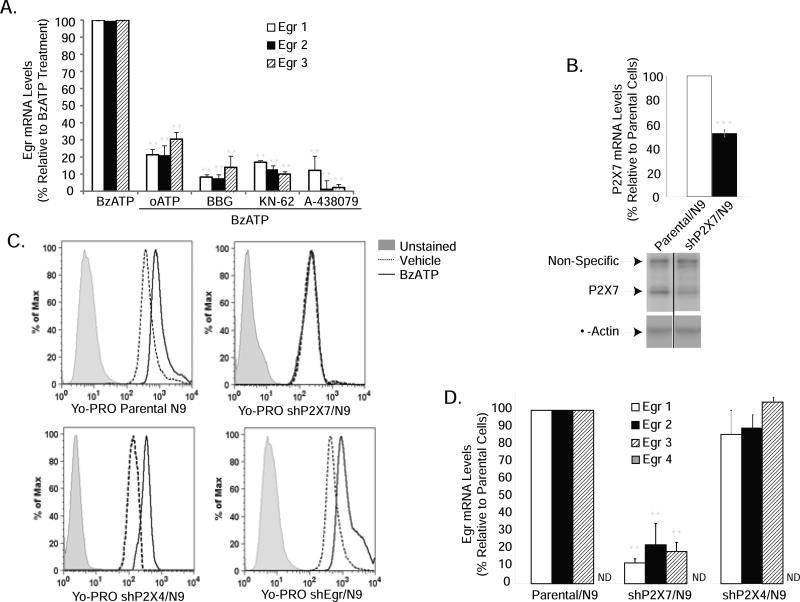
P2X7 receptors mediate the effects of BzATP on Egr induction
Although BzATP is a potent agonist of P2X7 receptors, it also has strong affinity for other P2X receptors (Bianchi et al. 1999; North 2002). Using the P2X7 antagonists oATP, BBG, KN-62 and A-438079, we evaluated the role of P2X7 receptors in Egr gene expression induced by BzATP. We found that all P2X7 antagonists significantly reduced the ability of BzATP to induce Egr factor expression by between 70% and 95% (Fig. 3A). We confirmed the role of P2X7 receptors in these effects of BzATP using RNAi. In N9 microglia stably expressing short hairpins to the P2X7 receptor (shP2X7), which show reduced P2X7 mRNA (Fig. 3B, graph), protein levels (Fig. 3B, blot) and functionality as assessed by reduced P2X7 pore activity (Fig. 3C, upper right panel), we found that BzATP-stimulated Egr gene induction was attenuated by approximately 80-90% (Fig. 3D), confirming the role of P2X7 receptors in these effects of BzATP. As a control for RNAi in these experiments, we knocked down P2X4 receptors since they are highly related to P2X7 receptors and BzATP is a potent agonist (Bianchi et al. 1999; North 2002). We find that the effects of BzATP on Egr gene induction in shP2X4/N9 cells are not different from those in parental cells (Fig. 3D). Egr-4 levels remained low/undetectable in both basal and BzATP-treated shP2X7- and shP2X4- expressing cells. P2X7 pore activity is unaffected by P2X4 knockdown (Fig. 3C, lower left panel). To determine if P2X4 receptors were functionally knocked down in shP2X4/N9 cells, we evaluated BDNF mRNA levels because P2X4 receptors are central to nucleotide-induced BDNF expression in microglia (Trang et al. 2009; Ulmann et al. 2008). We found that whereas BzATP-induced BDNF expression was comparable in parental and shP2X7/N9 cells, BDNF gene induction was not observed in shP2X4/N9 cells (Supplementary Fig. 2), indicating that P2X4 receptors are functionally reduced in these cells.
Figure 3. P2X7 purinergic receptors mediate induction of Egr factors by BzATP.
(A) P2X7 receptor antagonists significantly attenuate BzATP-induced Egr expression. N9 microglia were pre-treated with vehicle (250 mM Hepes) or the P2X7 receptor antagonists oATP (500μM) for 2 hours, or Brilliant Blue G (1mM) or KN-62 (25μM) for 30 minutes prior to stimulation with either vehicle or BzATP (150 μM) for 1 hour. The data shown represent the mean ± SEM of at least 3 independent experiments, expressed as percent gene induction relative to N9 cells treated with BzATP alone. (B) Cells stably expressing short hairpins to P2X7 receptors (shP2X7/N9) show decreased P2X7 mRNA (upper panel) and protein levels (lower panel). In the representative Western blot shown, a high molecular weight, non-specific band is observed using this P2X7 antibody. (C) YO-PRO uptake stimulated by BzATP in parental N9 cells (upper left panel) is abrogated in shP2X7/N9 (upper right panel), but not in shP2X4/N9 (lower left panel) or shEgr/N9 (lower right panel) cells. (D) BzATP-induced Egr expression is greatly attenuated in shP2X7/N9 but not in shP2X4/N9 cells treated with vehicle or BzATP for 1 hour. **p<0.01 vs. BzATP treated parental cells.
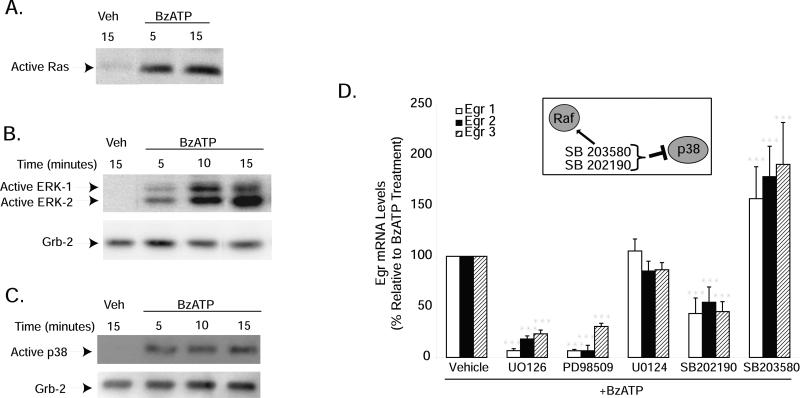
BzATP promotes Egr induction via activation of the ERK-1/-2 and p38 mitogen-activated protein kinase pathways in microglia
Egr factors are well-known to be regulated by MAP kinase pathways, especially by ERK-1/-2 (Lo et al. 2001; McCubrey et al. 2000; Park and Koh 1999); but the p38 pathway has also been shown to regulate Egr factors as well (Kim et al. 2007; Lim et al. 1998; Shin et al. 2006). To our knowledge, Ras activation in response to P2X7 receptor stimulation has only been demonstrated in RAW 264.7 macrophages (Aga et al. 2004), so we assessed its activation in microglia following treatment with BzATP. Ras is a small molecular weight G protein that lies upstream of MEK/ERK activation in many growth factor signaling pathways; we found that BzATP also promotes Ras activation within 5 minutes in microglia (Fig. 4A). Accordingly, BzATP rapidly increased ERK-1/-2 (Fig. 4B) and p38 (Fig. 4C) activation within 5-10 minutes of treatment, an effect that persisted at 15 minutes. Blockade of ERK-1/-2 activation using the MEK inhibitors PD98059 and U0126 (but not the inactive analog U0124), attenuated Egr gene induction stimulated by BzATP by about 70-90% (Fig. 4D). And interestingly, blockade of the p38 pathway with SB202190 also interfered with Egr induction stimulated by BzATP, but only by about 50%. In contrast, stimulation of Egr expression was observed using the p38 inhibitor SB203580. The concentrations of all inhibitors used in the present studies were those we have previously shown to be effective in preventing nucleotide and/or LPS signaling in microglia and macrophages (Baker et al. 2004; Watters et al. 2002). Although both the ERK and p38 MAP kinase pathways appear to play a role in the mechanisms whereby BzATP stimulates Egr expression, both pathways are not equally efficacious, since MEK inhibitors more strongly interfere with Egr induction than do p38 inhibitors. This idea is consistent with our previous studies wherein a role for the p38 MAP kinase pathway in the inhibitory effects of BzATP on NO production was not observed (Brautigam et al. 2005).
Figure 4. BzATP-induced Egr expression is sensitive to MEK and p38 inhibitors.
N9 microglia were treated with vehicle (250 mM Hepes) or BzATP (250μM) for the times indicated. Ras activation was assessed using a GST-Raf pull-down assay (A), and ERK-1/2 (B) and p38 (C) activation was detected using antibodies against the dually phosphorylated and enzymatically active proteins. (A) BzATP stimulates Ras activation within 5 minutes. BzATP-induced ERK-1/-2 (B) and p38 (C) activation is maximal within 5-10 minutes of treatment. Immunoreactivity of the cytosolic protein Grb-2 was used to verify protein loading. (D) Pharmacologic inhibitors of MEK and p38 interfere with BzATP-stimulated Egr induction. Cells were pre-treated with either vehicle (0.01% DMSO), the MEK inhibitors U0126 (10μM) or PD98509 (50μM), the inactive MEK inhibitor analog U0124 (10μM), or the p38 inhibitors SB202190 (10μM) or SB203580 (10μM) for 15 minutes prior to treatment with vehicle or BzATP (150μM) for 1 hour. Egr gene expression was assessed by qRT-PCR. Data are expressed as percent Egr expression relative to BzATP treatment alone and represent the mean of at least 3-8 independent experiments + SEM. ***p<0.001 vs. BzATP treatment.
The P2X7-Egr pathway is necessary for BzATP-mediated alterations in Egr target gene expression
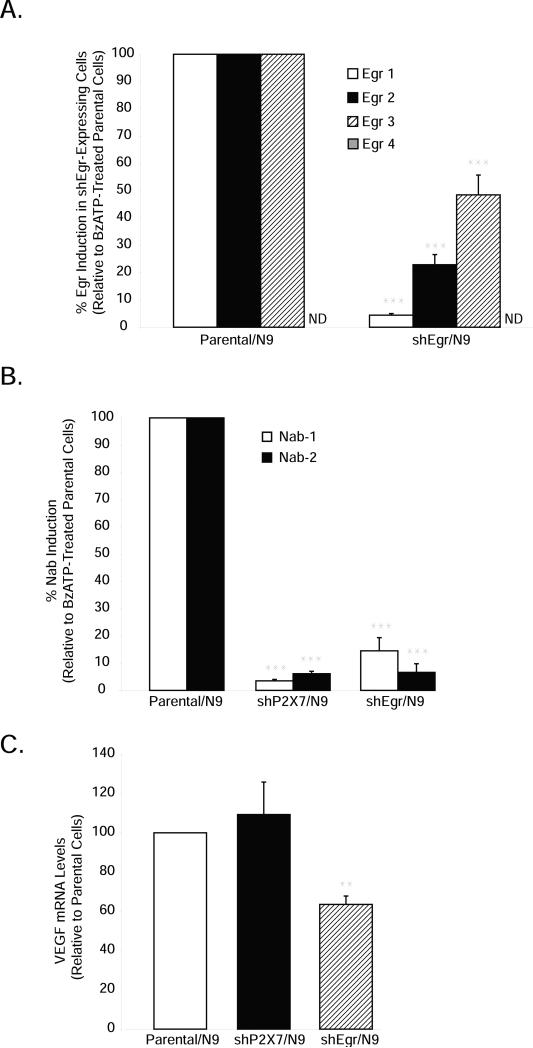
In order to specifically evaluate the role of Egr factors in P2X7-dependent signaling in microglia, we employed an RNAi strategy. Knock down of each Egr factor individually showed little, if any, alteration in BzATP-stimulated Egr target gene expression (data not shown), likely due to compensatory mechanisms by the remaining Egr factors. Therefore, we created N9 cells in which the levels of all three Egr factors detectably expressed in N9 microglia (Egr-1, -2 and -3) had been simultaneously knocked down (shEgr/N9). As shown in Figure 5A, in shEgr/N9 cells, we found BzATP-stimulated Egr-1 and Egr-2 expression to be decreased by 80-95%, whereas that of Egr-3 was reduced by about 50%. P2X7 receptor function was intact in these cells because BzATP-mediated YO-PRO uptake was not different from that in parental N9 cells (Fig. 3D, lower right panel). Egr-4 levels in shEgr/N9 cells remained unchanged and were still very lowly detectable (Fig. 5A) even when their Egr family counterparts are knocked down. We next assessed if knockdown of Egr expression in these cells translated into functional reductions in Egr activity in cells expressing short hairpins to Egr factors and P2X7. To do this, we evaluated the expression of the known Egr target genes NGFI-A binding proteins -1 and -2 (NAB-1 and NAB-2) (Mechta-Grigoriou et al. 2000; Nagarajan et al. 2001), which are not readily detectable in our cells in the absence of stimulation. In shP2X7-expressing cells, NAB-1 and NAB-2 expression was almost completely abolished (<5% expression remained) whereas in shEgr-expressing cells treated with BzATP, NAB-1 and NAB-2 gene expression was reduced by 85-95% (Fig. 5B), suggesting that BzATP regulates NAB expression via P2X7 receptor-Egr pathway activation, and that our Egr hairpins effectively reduce Egr function. As another control for functional Egr knockdown, we also evaluated the basal expression of vascular endothelial growth factor (VEGF), a cytokine known to be positively regulated by Egr factors (Worden et al. 2005), but not by P2X7 receptors in immune cells (Wei et al. 2008). Whereas RNAi to P2X7 receptors had no effect, shEgr expression significantly reduced VEGF mRNA levels (Fig. 5C).
Figure 5. BzATP-mediated Egr target gene induction is dependent on the P2X7-Egr pathway.
Parental N9, shEgr/N9 or shP2X7/N9 microglia were treated with either vehicle (250 mM Hepes) or BzATP (150μM) for 1 hour (A, B) or 20 hours (C). (A) BzATP-induced Egr expression is blunted in shEgr/N9 cells. (B) RNAi to P2X7 and Egr factors prevents BzATP-induced Egr target gene (Nab-1/Nab-2) activation. Nab gene induction by BzATP in parental cells ranged from 3-14 fold over vehicle treatment. (C) RNAi to Egr factors, but not to P2X7 receptors, decreases VEGF expression. The data are graphed as fold induction relative to BzATP-treated parental N9 cells for each gene and represent the means of 4-8 independent experiments + SEM.
P2X7 receptors are necessary for the effects of BzATP on LPS-stimulated iNOS and TNF-α production
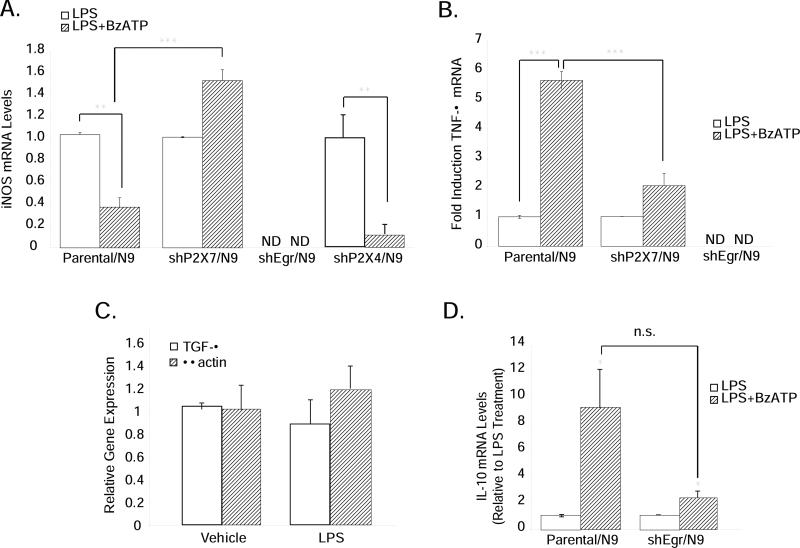
We next returned to the original question, which was to determine if the P2X7-Egr pathway was involved in the inhibitory effects of BzATP on iNOS gene expression. We found that in shP2X7-expressing cells, but not in shP2X4/N9 cells, the inhibitory effects of BzATP on iNOS expression were abrogated (Fig. 6A), confirming the involvement of P2X7. Surprisingly however, in cells expressing short hairpins to Egr factors, iNOS mRNA levels were not detectable in the LPS- or BzATP-treated state, suggesting that Egr factors may be necessary for basal iNOS expression levels as well as for P2X7 receptor-dependent regulation.
Figure 6. BzATP-induced alterations in LPS-stimulated NO and TNF-α production requires the P2X7-Egr pathway.
Parental N9, shP2X7/N9 and shEgr/N9 cells were treated with vehicle (250 mM Hepes), LPS (1 μg/mL), or LPS+BzATP (150 μM) overnight. (A) BzATP-mediated inhibition of LPS-stimulated iNOS expression does not occur in shP2X7/N9 and shEgr/N9 cells. (B) BzATP-mediated augmentation of LPS-induced TNF-α expression is abrogated in shP2X7/N9 and shEgr/N9 cells. (C) Expression of TGF-β and β-actin are unchanged in shEgr/N9 cells treated with vehicle or LPS. (D) BzATP treatment increases LPS-stimulated IL-10 mRNA levels in both parental N9 and shEgr/N9 cells. The means ± SEM are graphed as fold induction relative to vehicle treatment (C) or LPS treatment (A, B, D) in 3-8 independent experiments. * p<0.05, **p<0.01, ***p<0.001 vs. vehicle or LPS treatment.
Because TNF-α expression is positively regulated by Egr factors, we reasoned that if P2X7 receptors promoted Egr induction in a meaningful way in microglia, and Egr factors increase TNF-α expression, then BzATP treatment via induction of Egr factors would also augment LPS-stimulated TNF-α expression. As shown in Figure 6B, BzATP strongly increases LPS-stimulated TNF-α expression in parental cells, an effect which is significantly attenuated in shP2X7/N9 and shEgr/N9 cells. However, similar to iNOS expression, we found that basal and LPS-stimulated TNF-α mRNA levels were also not detectable in shEgr/N9 cells. But shEgr/N9 cells are viable and healthy by all endpoints we have evaluated; the expression of short hairpins to Egr factors does not universally decrease or ablate the expression of all genes in the presence of LPS. For example, in shEgr/N9 cells the levels of transforming growth factor (TGF)-β (an Egr target gene in several tumor cell types (Liu et al. 1996a; Liu et al. 1996b) but not known to be transcriptionally regulated by LPS), and β-actin (the expression of which is commonly evaluated as a standard “housekeeping” gene in experiments like these) are not different in the presence or absence of LPS (Fig. 6C). In addition, the effects of BzATP on LPS-stimulated IL-10 expression, a transcriptional target gene of LPS signaling (Aloisi et al. 1999), which is not known to be transcriptionally regulated by Egr factors, were not different between parental/N9 and shEgr/N9 cells (Fig. 6D), indicating that both LPS and BzATP have the capacity to influence gene expression normally in shEgr/N9 cells as long as functional Egr factors are not required.
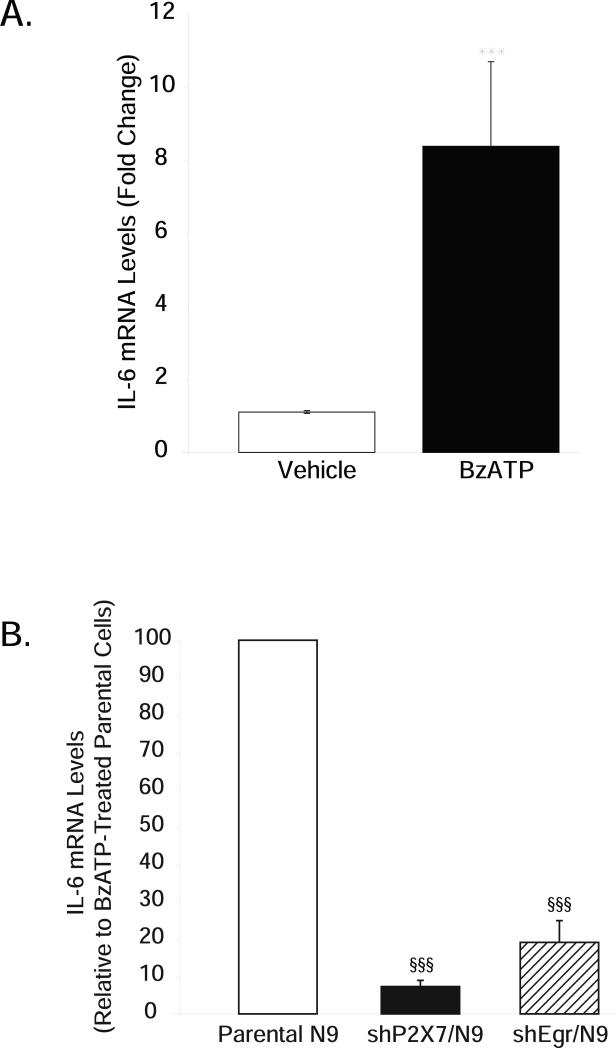
RNAi to P2X7 and Egr factors abrogate BzATP-induced IL-6 production
Given that our ability to interpret the role of the P2X7-Egr pathway in regulating iNOS and TNFα inflammatory gene expression in microglia was confounded by LPS, we next chose to ascertain the role of P2X7 receptor activation alone on microglial gene expression. However, this is difficult because there are few reports in the literature indicating effects of P2X7 receptor activation on inflammatory mediator production in cells that have not been previously primed with an activating stimulus like LPS, beta amyloid peptides or hypoxia (Brautigam et al. 2005; Morigiwa et al. 2000; Ogata et al. 2003; Rampe et al. 2004). Moreover, the few reports that do indicate that P2X7 receptor activation (by itself) promotes inflammatory gene expression, demonstrate effects that do not occur in the microglia we use. For example, BzATP reportedly increases TNF-α release and transcription in primary rat microglia (Hide et al. 2000) but we do not observe any effects of BzATP alone on TNF-α in either murine N9 microglia or in primary mouse microglia (data not shown); this could be a species difference.
Therefore, we chose to evaluate the effects of P2X7 receptors on the expression of the Th2 cytokine IL-6 because available information indicates an effect of P2X7 receptors on IL-6 release (Boucsein et al. 2003; Rampe et al. 2004; Shigemoto-Mogami et al. 2001), and IL-6 expression is regulated by Egr factors in other cell types (Hoffmann et al. 2008; Prince et al. 2007; Worden et al. 2005). Although effects on transcription were not evaluated, based on these data, and our observations that P2X7 receptors stimulate Egr factors in microglia, we reasoned that if activation of the P2X7-Egr pathway is sufficient for promoting inflammatory gene transcription, then P2X7 receptor activation alone would increase IL-6 production in microglia. Indeed, we found that BzATP promoted IL-6 expression (Fig. 7A) in the absence of any other microglial stimuli, and that IL-6 mRNA levels were greatly attenuated in cells expressing short hairpins both to P2X7 and Egr factors (Fig. 7B). Importantly, basal IL-6 expression remains detectable in shEgr/N9 cells. Approximately 10-20% of BzATP-stimulated IL-6 mRNA levels remain in both shP2X7/N9 and shEgr/N9 cells. These data further support the contention that ablation of both basal and LPS-stimulated iNOS and TNF-α levels in shEgr/N9 cells are likely to be gene-specific effects. They also identify IL-6 as a novel transcriptional target of the P2X7-Egr factor pathway in microglia.
Figure 7. BzATP-induced IL-6 expression is blocked in microglia stably expressing short hairpins to P2X7 and Egr factors.
Parental N9, shP2X7/N9 and shEgr/N9 cells were treated overnight with either vehicle (250 mM Hepes) or BzATP (150 μM). (A) BzATP increases IL-6 expression in parental N9 microglia. (B) BzATP-induced IL-6 expression is strongly attenuated in shP2X7/N9 and shEgr/N9 cells. The data are graphed as fold induction relative to either vehicle (A) or BzATP treatment (B) in each cell type. Treatments represent the mean ± SEM of at least 4 independent experiments. ***p<0.001 vs. vehicle treatment; §§§p<0.001 vs. BzATP-treated parental cells.
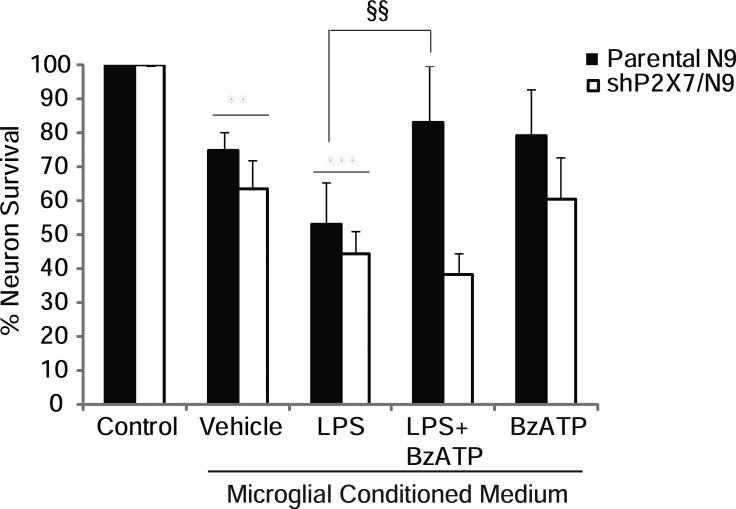
BzATP confers protection from LPS-induced hippocampal neuron death via P2X7 receptors
To determine if the effects of BzATP on altered microglial inflammatory gene expression is functionally relevant to neurons, we performed cell survival studies on hippocampal HT22 neurons using microglial conditioned medium. Conditioned medium (CM) from LPS-treated parental (black bars) and shP2X7/N9 (white bars) microglial cell lines induced significant (~50%) neuronal death (Fig. 8). However, CM from parental N9 cells treated with both LPS and BzATP together induced significantly less death than LPS CM, suggesting that BzATP reduces microglial production of neurotoxic, or increases the production of neuroprotective factors. Interestingly, neuronal death induced by LPS CM was unchanged in the presence of BzATP when the CM was from shP2X7/N9 cells, suggesting that the neuroprotective effects of BzATP are mediated by P2X7 receptors. Although neuronal death was observed in CM from BzATP-treated microglia, this was not statistically different from that promoted by CM from vehicle-treated cultures, and cell death induced by LPS CM was significantly greater than that induced by vehicle or BzATP CM. Decreased neuron survival resulting from the addition of CM from unactivated microglia to neuronal cultures has also been previously reported (Ciesielski-Treska et al. 2001; Hur et al. ; Wu et al. 2000).
Figure 8. BzATP abrogates LPS-induced HT22 hippocampal neuron death via P2X7 receptors.
Parental N9 and shP2X7/N9 cells were treated overnight with vehicle (250 mM Hepes) or LPS (1 μg/mL) in the presence and absence of BzATP (150 μM). Microglial conditioned medium was applied to HT22 neurons for 18-22 hours after which time neuron survival was assessed by the MTS mitochondrial function assay. The data are graphed as % of neurons surviving relative to control treatment and represent the means of 3-6 independent experiments ± SEM. ** p<0.01 vs. control; ***p<0.001 vs. control; §§ p<0.01 vs. LPS.
Discussion
In the present studies, we report for the first time that P2X7 receptor activation promotes induction of Egr-1, -2 and -3 transcription factors in microglia, and that these factors are necessary for P2X7-induced IL-6 gene expression as well as modulation of LPS-stimulated iNOS and TNF-α expression by P2X7 receptors. To our knowledge, there have been no previous reports of P2X7 receptor activation exerting effects on Egr factors in any immune cell type. The two available studies in this regard show activation of Egr-1 indirectly by P2X7 receptors in adventitial fibroblasts exposed to hypoxia (Gerasimovskaya et al. 2002) or by BzATP treatment of HEK 293 cells transiently transfected with P2X7 receptors (Stefano et al. 2007); Egr-1 was the only Egr factor evaluated in both studies.
Although the P2X7 receptor is one of the best studied P2 receptors, there is surprisingly little information available about how its activation regulates gene transcription in any cell type. Reports of P2X7-dependent transcription factor activation are plentiful, and many correlative studies exist discussing the potential significance of transcription factor activation following P2X7 activation. However, important with reference to the present studies, only one paper to our knowledge has directly demonstrated the necessity of a transcription factor activated by P2X7 receptors (NFAT) in controlling subsequent gene transcription (Kataoka et al. 2009). This very recent paper demonstrated that P2X7 receptor-dependent NFAT activation promotes MIP-1α expression in microglia, which may contribute to T cell recruitment to the CNS in certain neurodegenerative disorders.
Another important finding in the present report is the demonstration that P2X7 receptors have differential effects on the expression of different LPS-stimulated inflammatory genes. For example, P2X7 activation decreases LPS-induced iNOS expression and NO production, whereas it increases that of TNF-α. In addition, activation of P2X7 receptors in quiescent or unactivated microglia promotes the expression of IL-6, but has no detectable effect on TNF-α or iNOS gene expression in the absence of microglial activating stimuli. Our data also show that P2X7 receptor activation attenuates LPS-induced neuronal death, possibly representing a neuroprotective response of P2X7 receptors. Interestingly, that several reports demonstrate neuroprotective effects of TNF-α and IL-6 (reviewed in (Morganti-Kossmann et al. 2007; Stoll et al. 2000)), two inflammatory genes whose expression we find to be augmented by P2X7 receptors. The reduction in LPS-stimulated NO release as a result of P2X7 receptor activation may also be neuroprotective, but NO in its own right has also been suggested to exert neuroprotective effects in certain circumstances (Chiueh 1999). These results raise the exciting possibility that selective activation of P2X7 receptors may promote neuroprotection in certain circumstances, but further studies are necessary to address this fully.
Consistent with reports of Egr induction in other cell types, we find that P2X7-dependent Egr gene stimulation in microglia is also dependent on MAP kinase activation. In particular, ERK-1/-2 appear to have dominant effects on Egr induction in response to BzATP treatment, although p38 also plays a role. It is necessary to remark here however, that the two different p38 inhibitors we used in our studies gave opposing effects; SB202190 attenuated Egr induction whereas SB203580 potentiated it, consistent with other Egr reports using the SB203580 inhibitor (Rolli-Derkinderen and Gaestel 2000; Schaefer et al. 2004). We surmise that augmented Egr expression in the presence of SB203580 likely involves its ability to stimulate Raf kinase activity (Yoon et al. 2004), an effect that is not well-referenced in the Egr or signaling literature to our knowledge.
It was unanticipated that short hairpins to Egr factors would interfere with basal iNOS expression and gene induction by LPS since we have been unable to find any reports indicating direct regulation of the iNOS gene by Egr factors in any cell type. However, Egr factors are central to LPS stimulation of other inflammatory genes (Pawlinski et al. 2003; Shi et al. 2002), and we have identified several putative DNA binding sites for Egr factors in the 5’flanking region of the iNOS gene which may underlie the present observations. In support of this notion, Harada et al. recently demonstrated that iNOS expression is decreased in lung epithelial allografts from Egr-1 knockout mice compared to wildtype mice (Harada et al. 2007), suggesting that the absence of Egr-1 negatively influences iNOS expression. Although a direct role for transcriptional regulation of iNOS by Egr factors was surmised because of two Egr binding sites identified in the iNOS promoter by another group, details were not provided. Our own data are also consistent with this notion; we find that basal iNOS levels in microglia isolated from Egr-1 knockout mice are considerably lower than in microglia from wild type mice (unpublished observations, SAF, JPS and JJW). Further studies to assess the means whereby Egr factors regulate basal and/or LPS-stimulated iNOS gene expression are necessary to better understand the complex regulation of this gene. The ablation of basal TNF-α expression in shEgr/N9 cells was similarly unexpected, but the critical role of Egr factors in mediating the stimulatory effects of LPS on TNF-α expression are well-documented (Tsai et al. 2000; Yao et al. 1997).
Because we found that both basal and LPS-stimulated expression of iNOS and TNF-α were absent in cells stably expressing short hairpins to Egr factors, it was possible that gene expression in general, or specifically in response to LPS treatment, was somehow aberrant in these cells. To investigate this, we examined TGF-β and β-actin gene expression in the presence and absence of LPS. TGF-β is a known Egr target gene in certain tumor cell types (Liu et al. 1996a), but to our knowledge it is not a known target of LPS action in macrophage-like cells. We also evaluated the expression of β-actin because this gene is commonly used to normalize mRNA content between samples in RT-PCR, and its expression is not known to be regulated by Egr factors or LPS in microglia. We found that the levels of TGF-β and β-actin were not different in shEgr/N9 cells treated with vehicle or LPS, suggesting that LPS treatment of these cells does not universally decrease or ablate the expression of all genes. To ensure that LPS and P2X7 receptor signaling was intact in shEgr/N9 cells, we had to identify another target gene whose LPS-induced expression was changed by BzATP in a manner that was independent of Egr factors; there is no literature in this regard. P2X7 receptors were suggested to play a role in regulating IL-10 levels because inflammation-induced IL-10 levels were reduced in P2X7 receptor knockout mice (Chessell et al. 2005), and other studies demonstrated that BzATP was the most potent P2 receptor agonist tested at promoting IL-10 expression in cultured rat microglia (Seo et al. 2008), though the effects of BzATP were independent of P2X7 receptors. Therefore, we reasoned at a minimum that we would obtain a response to BzATP treatment on LPS-stimulated IL-10 expression, an effect of LPS and BzATP that would likely be independent of Egr signaling. We found that while BzATP significantly augmented LPS-induced IL-10 expression in both parental and shEgr/N9 microglia, suggesting that LPS and BzATP signaling in both cell lines is comparable. The smaller variation in the magnitude of the BzATP effects on LPS-induced IL-10 expression observed in the shEgr/N9 cells compared to the parental cells is likely the result of population selection during preparation of the stable shEgr/N9 cell line.
Many of the activities assigned to P2X7 receptors are based primarily on the use of two pharmacologic agents: 3’-O-(4-benzoylbenzoyl)-adenosine 5’-triphosphate (BzATP), a strong agonist of P2X7 receptors but a more potent agonist of other P2X receptor subtypes (Burnstock and Knight 2004; North 2002), and oxidized ATP, a P2X7 receptor antagonist that also has effects independent of P2X7 receptors (Beigi et al. 2003). In our study, we used complementary pharmacologic and RNAi approaches to overcome these issues, and to test the role of P2X7 receptors in the regulation of Egr and inflammatory gene transcription. We describe here a new pathway whereby P2X7 receptors utilize Egr transcription factors to differentially regulate the expression of important microglial inflammatory genes. Because the inappropriate activation of microglia and their production of neurotoxic cytokines, and reactive oxygen and nitrite species are thought to play a role in the pathology of many neurodegenerative disorders, agents that function to reduce microglial pro-inflammatory activities and/or stimulate their anti-inflammatory/neuroprotective activities are being actively sought. We postulate that the P2X7-Egr pathway may be one such target in microglia that could be used to modulate their expression of factors such as NO, TNF-α and IL-6, molecules that can exert condition-specific, pleiotropic and potentially neuroprotective effects in the CNS.
Supplementary Material
Acknowledgements
We would like to thank Dr. John Svaren for thoughtful discussion and advice regarding the Egr factors and Ms. Chuenchanok Frasier for excellent technical assistance. This work was supported by the NIH/NINDS R01NS049033, the University of Wisconsin Alumni Research Foundation, and the NIH Training Grants T32HL007654 (SAF) and K12AG019247, T32HL007654 (MN).
References
- Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, Bertics PJ, Darien BJ. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7). Journal of Leukocyte Biology. 2002;72(1):222–32. [PubMed] [Google Scholar]
- Aga M, Watters JJ, Pfeiffer ZA, Wiepz GJ, Sommer JA, Bertics PJ. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-{kappa}B signaling pathways in murine RAW 264.7 macrophages. Am J Physiol Cell Physiol. 2004;286(4):C923–930. doi: 10.1152/ajpcell.00417.2003. [DOI] [PubMed] [Google Scholar]
- Aloisi F, De Simone R, Columba-Cabezas S, Levi G. Opposite effects of interferon-gamma and prostaglandin E2 on tumor necrosis factor and interleukin-10 production in microglia: a regulatory loop controlling microglia pro- and anti-inflammatory activities. Journal of Neuroscience Research. 1999;56(6):571–80. doi: 10.1002/(SICI)1097-4547(19990615)56:6<571::AID-JNR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen Modulates Microglial Inflammatory Mediator Production via Interactions with Estrogen Receptor {beta}. Endocrinology. 2004;145(11):5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol. 2003;140(3):507–19. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. Journal of Neuroscience. 1998;18(5):1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. European Journal of Pharmacology. 1999;376(1-2):127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17(11):2267–76. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- Brautigam VM, Frasier C, Nikodemova M, Watters JJ. Purinergic receptor modulation of BV-2 microglial cell activity: Potential involvement of p38 MAP kinase and CREB. Journal of Neuroimmunology. 2005;166(1-2):113. doi: 10.1016/j.jneuroim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular Distribution and Functions of P2 Receptor Subtypes in Different Systems International Review of Cytology. Academic Press; 2004. pp. 31–304. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386. doi: 10.1016/j.pain.2005.01.002. others. [DOI] [PubMed] [Google Scholar]
- Chiueh CC. Neuroprotective properties of nitric oxide. Ann N Y Acad Sci. 1999;890:301–11. doi: 10.1111/j.1749-6632.1999.tb08007.x. [DOI] [PubMed] [Google Scholar]
- Ciesielski-Treska J, Ulrich G, Chasserot-Golaz S, Zwiller J, Revel M-O, Aunis D, Bader M-F. Mechanisms Underlying Neuronal Death Induced by Chromogranin A-activated Microglia. Journal of Biological Chemistry. 2001;276(16):13113–13120. doi: 10.1074/jbc.M009711200. [DOI] [PubMed] [Google Scholar]
- Decker EL, Nehmann N, Kampen E, Eibel H, Zipfel PF, Skerka C. Early growth response proteins (EGR) and nuclear factors of activated T cells (NFAT) form heterodimers and regulate proinflammatory cytokine gene expression. Nucleic Acids Research. 2003;31(3):911–21. doi: 10.1093/nar/gkg186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. Journal of Biological Chemistry. 1999;274(19):13205–10. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. Journal of Immunology. 1996;156(4):1531–9. [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. Journal of Cell Biology. 1997;139(7):1635–43. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP Is an Autocrine/Paracrine Regulator of Hypoxia-induced Adventitial Fibroblast Growth. SIGNALING THROUGH EXTRACELLULAR SIGNAL-REGULATED KINASE-1/2 AND THE Egr-1 TRANSCRIPTION FACTOR. J Biol Chem. 2002;277(47):44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107(12):4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- Harada H, Lama VN, Badri LN, Ohtsuka T, Petrovic-Djergovic D, Liao H, Yoshikawa Y, Iwanaga K, Lau CL, Pinsky DJ. Early growth response gene-1 promotes airway allograft rejection. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L124–130. doi: 10.1152/ajplung.00285.2006. [DOI] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. Journal of Neurochemistry. 2000;75(3):965–72. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Hipskind RA, Baccarini M, Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol. 1994;14(9):6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Ashouri J, Wolter S, Doerrie A, Dittrich-Breiholz O, Schneider H, Wagner EF, Troppmair J, Mackman N, Kracht M. Transcriptional Regulation of EGR-1 by the Interleukin-1-JNK-MKK7-c-Jun Pathway. J Biol Chem. 2008;283(18):12120–12128. doi: 10.1074/jbc.M800583200. [DOI] [PubMed] [Google Scholar]
- Hur J, Lee P, Kim MJ, Kim Y, Cho Y-W. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochemical and Biophysical Research Communications. 2010;391(3):1526–1530. doi: 10.1016/j.bbrc.2009.12.114. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, Kohsaka S. ATP stimulation of Ca2+ -dependent plasminogen release from cultured microglia. British Journal of Pharmacology. 1998;123(7):1304–10. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108(1):115–25. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- Kim CG, Choi BH, Son SW, Yi SJ, Shin SY, Lee YH. Tamoxifen-induced activation of p21Waf1/Cip1 gene transcription is mediated by Early Growth Response-1 protein through the JNK and p38 MAP kinase/Elk-1 cascades in MDA-MB-361 breast carcinoma cells. Cell Signal. 2007;19(6):1290–300. doi: 10.1016/j.cellsig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lim CP, Jain N, Cao X. Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1. Oncogene. 1998;16(22):2915–26. doi: 10.1038/sj.onc.1201834. [DOI] [PubMed] [Google Scholar]
- Liu C, Adamson E, Mercola D. Transcription factor EGR-1 suppresses the growth and transformation of human HT-1080 fibrosarcoma cells by induction of transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1996a;93(21):11831–6. doi: 10.1073/pnas.93.21.11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Calogero A, Ragona G, Adamson E, Mercola D. EGR-1, the reluctant suppression factor: EGR-1 is known to function in the regulation of growth, differentiation, and also has significant tumor suppressor activity and a mechanism involving the induction of TGF-beta1 is postulated to account for this suppressor activity. Crit Rev Oncog. 1996b;7(1-2):101–25. [PubMed] [Google Scholar]
- Lo LW, Cheng JJ, Chiu JJ, Wung BS, Liu YC, Wang DL. Endothelial exposure to hypoxia induces Egr-1 expression involving PKCalpha-mediated Ras/Raf-1/ERK1/2 pathway. Journal of Cellular Physiology. 2001;188(3):304–12. doi: 10.1002/jcp.1124. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, May WS, Duronio V, Mufson A. Serine/threonine phosphorylation in cytokine signal transduction. Leukemia. 2000;14(1):9–21. doi: 10.1038/sj.leu.2401657. [DOI] [PubMed] [Google Scholar]
- Mechta-Grigoriou F, Garel S, Charnay P. Nab proteins mediate a negative feedback loop controlling Krox-20 activity in the developing hindbrain. Development. 2000;127(1):119–28. doi: 10.1242/dev.127.1.119. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Kohlhaas KL, Matsumoto T, Pollock JS, Forstermann U, Warner TD, Schmidt HH, Murad F. Induction of NADPH-dependent diaphorase and nitric oxide synthase activity in aortic smooth muscle and cultured macrophages. Molecular Pharmacology. 1992;41(6):1163–8. [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38(12):1392–400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 Purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neuroscience Letters. 2000;282(3):153–6. doi: 10.1016/s0304-3940(00)00887-9. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30(2):355–68. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaB alpha degradation in a stimulus-specific manner in microglia. Journal of Neurochemistry. 2006;96(2):314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular Physiology of P2X Receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ogata T, Chuai M, Morino T, Yamamoto H, Nakamura Y, Schubert P. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Research. 2003;981(1-2):174. doi: 10.1016/s0006-8993(03)03028-2. [DOI] [PubMed] [Google Scholar]
- Park JA, Koh JY. Induction of an immediate early gene egr-1 by zinc through extracellular signal-regulated kinase activation in cortical culture: its role in zinc-induced neuronal death. Journal of Neurochemistry. 1999;73(2):450–6. doi: 10.1046/j.1471-4159.1999.0730450.x. [DOI] [PubMed] [Google Scholar]
- Pawlinski R, Pedersen B, Kehrle B, Aird WC, Frank RD, Guha M, Mackman N. Regulation of tissue factor and inflammatory mediators by Egr-1 in a mouse endotoxemia model. Blood. 2003;101(10):3940–7. doi: 10.1182/blood-2002-07-2303. [DOI] [PubMed] [Google Scholar]
- Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP Acts as an Agonist to Promote Stimulus-Induced Secretion of IL-1{beta} and IL-18 in Human Blood. J Immunol. 2000;165(8):4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochemistry International. 2006;49:204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Prince JM, Ming MJ, Levy RM, Liu S, Pinsky DJ, Vodovotz Y, Billiar TR. Early growth response 1 mediates the systemic and hepatic inflammatory response initiated by hemorrhagic shock. Shock. 2007;27(2):157–64. doi: 10.1097/01.shk.0000245025.01365.8e. [DOI] [PubMed] [Google Scholar]
- Rampe D, Wang L, Ringheim GE. P2X7 receptor modulation of [beta]-amyloid- and LPS-induced cytokine secretion from human macrophages and microglia. Journal of Neuroimmunology. 2004;147(1-2):56. doi: 10.1016/j.jneuroim.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. European Journal of Immunology. 1989;19(8):1443–8. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Rolli-Derkinderen M, Gaestel M. p38/SAPK2-dependent gene expression in Jurkat T cells. Biol Chem. 2000;381(3):193–8. doi: 10.1515/BC.2000.026. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Kósa F, Bittorf T, Magócsi M, Rosche A, Ramirez-Chávez Y, Marotzki S, Marquardt H. Opposite effects of inhibitors of mitogen-activated protein kinase pathways on the egr-1 and [beta]-globin expression in erythropoietin-responsive murine erythroleukemia cells. Cellular Signalling. 2004;16(2):223. doi: 10.1016/j.cellsig.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Seo DR, Kim SY, Kim KY, Lee HG, Moon JH, Lee JS, Lee SH, Kim SU, Lee YB. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp Mol Med. 2008;40(1):19–26. doi: 10.3858/emm.2008.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Kishore R, McMullen MR, Nagy LE. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. American Journal of Physiology - Cell Physiology. 2002;282(6):C1205–11. doi: 10.1152/ajpcell.00511.2001. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. Journal of Neurochemistry. 2001;78(6):1339–49. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Shin SY, Lee JH, Min B, Lee YH. The translation inhibitor anisomycin induces Elk-1-mediated transcriptional activation of egr-1 through multiple mitogen-activated protein kinase pathways. Exp Mol Med. 2006;38(6):677–85. doi: 10.1038/emm.2006.80. [DOI] [PubMed] [Google Scholar]
- Stefano L, Rossler OG, Griesemer D, Hoth M, Thiel G. P2X(7) receptor stimulation upregulates Egr-1 biosynthesis involving a cytosolic Ca(2+) rise, transactivation of the EGF receptor and phosphorylation of ERK and Elk-1. J Cell Physiol. 2007;213(1):36–44. doi: 10.1002/jcp.21085. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. Journal of Neural Transmission Supplementum. 2000;59:81–9. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29(11):3518–28. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A Lipopolysaccharide-Specific Enhancer Complex Involving Ets, Elk-1, Sp1, and CREB Binding Protein and p300 Is Recruited to the Tumor Necrosis Factor Alpha Promoter In Vivo. Mol Cell Biol. 2000;20(16):6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–8. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JJ, Sommer JA, Fisette PL, Pfeiffer ZA, Aga M, Prabhu U, Guerra A, Denlinger LC, Bertics PJ. The P2X7 nucleotide receptor: Modulation of LPS-induced macrophage signaling and mediator production. Drug Develop Res. 2001;53:91–104. [Google Scholar]
- Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu U, Guerra AN, Bertics PJ. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1beta production. Journal of Biological Chemistry. 2002;277(11):9077–87. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- Wei W, Ryu JK, Choi HB, McLarnon JG. Expression and function of the P2X7 receptor in rat C6 glioma cells. Cancer Letters. 2008;260(1-2):79. doi: 10.1016/j.canlet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Williams LM, Lali F, Willetts K, Balague C, Godessart N, Brennan F, Feldmann M, Foxwell BMJ. Rac mediates TNF-induced cytokine production via modulation of NF-[kappa]B. Molecular Immunology. 2008;45(9):2446. doi: 10.1016/j.molimm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Worden B, Yang XP, Lee TL, Bagain L, Yeh NT, Cohen JG, Van Waes C, Chen Z. Hepatocyte growth factor/scatter factor differentially regulates expression of proangiogenic factors through Egr-1 in head and neck squamous cell carcinoma. Cancer Res. 2005;65(16):7071–80. doi: 10.1158/0008-5472.CAN-04-0989. [DOI] [PubMed] [Google Scholar]
- Wu Q, Combs C, Cannady SB, Geldmacher DS, Herrup K. Beta-amyloid activated microglia induce cell cycling and cell death in cultured cortical neurons. Neurobiology of Aging. 2000;21(6):797–806. doi: 10.1016/s0197-4580(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272(28):17795–801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R, Choi EJ, Yoo YS. SB203580 induces prolonged B-Raf activation and promotes neuronal differentiation upon EGF treatment of PC12 cells. Biochemistry (Mosc) 2004;69(7):799–805. doi: 10.1023/b:biry.0000040206.71415.6f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.