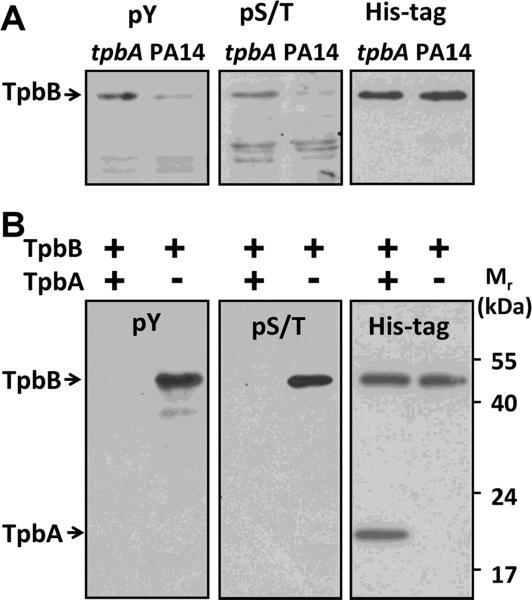
Figure 2.
Western blot analysis of TpbB phosphorylation in vivo and in vitro. (A) Whole membrane proteins were isolated from PA14 wild-type cells and from the tpbA mutant. TpbB with a C-terminal His-tag was produced in these cells by the pMQ70-tpbB plasmid. Proteins in the blot were detected by a phospho-Tyr antibody 4G10 (pY) or a phospho-Ser/Thr antibody (pS/T). The same membrane was then stripped and proteins were detected by a His-tag antibody to confirm the same amount of TpbB was produced and loaded for each sample as shown in the right blot.
(B) TpbB was incubated with or without TpbA at 37°C for 30 min; the reaction was quenched by adding SDS-loading dye and incubating at 95°C for 10 min. The reaction product was resolved by 10% SDS-PAGE and detected using the same antibodies as (A). Each experiment was performed twice, and a representative blot is shown.