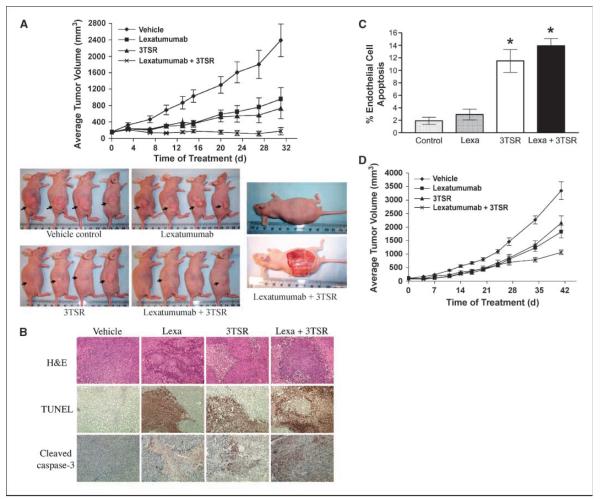
Figure 1.
3TSR and TRAIL cooperate in inhibiting colon cancer. A, human colon cancer SW480 cells were implanted into the right flankof nu/nu mice. Seven days post-implantation, mice were injected daily with Lexatumumab (3 mg/kg intravenously) and 3TSR (1 mg/kg intraperitoneally) or 0.9% saline and TSR buffer. Mean from 10 mice in each group (n = 10). By treatment day 31, ANOVA indicates P < 0.01 for Lexatumumab versus vehicle, P < 0.001 for 3TSR versus vehicle, P < 0.001 for Lexatumumab + 3TSR versus vehicle, and P < 0.05 for Lexatumumab + 3TSR versus Lexatumumab. By day 31, TGI = 60% for Lexatumumab, TGI = 69% for 3TSR, and TGI = 93% for Lexatumumab + 3TSR. B, immunohistochemical staining on SW480 tumor sections for TUNEL and cleaved caspase-3. SW480 xenograft tumors were collected from three mice from each treatment group on day 28 after tumor cell inoculation. Similar results were observed from tumor samples of three mice from each treatment group. Original photos were taken at ×200 magnification. C, analysis of tumor vasculature endothelial cell apoptosis. The apoptosis of tumor-associated vascular endothelial cells was evaluated via double labeling of CD31 and TUNEL. 3TSR significantly induced apoptosis of tumor vascular endothelial cells (P = 0.001 versus vehicle control), whereas Lexatumumab (Lexa) treatment showed little effect on tumor vascular endothelial cell apoptosis. The combination of Lexatumumab and 3TSR resulted in a similar level of endothelial cell apoptosis as with 3TSR alone (*, P < 0.001 versus control). D, growth of HCT116 colon cancer xenograft in nude mice. Tumor implantation and treatments were same as A, except 1 × 106 cells were used for implantation. Points, mean from 12 mice in each group (n = 12). By treatment day 41, statistical analysis by ANOVA indicates P < 0.01 for ×Lexatumumab versus vehicle, P < 0.05 for 3TSR versus vehicle, P < 0.001 for Lexatumumab + 3TSR versus vehicle, and P < 0.05 for Lexatumumab + 3TSR versus 3TSR. By treatment day 41, TGI = 46% for Lexatumumab, TGI = 36% for 3TSR, and TGI = 68% for Lexatumumab + 3TSR.