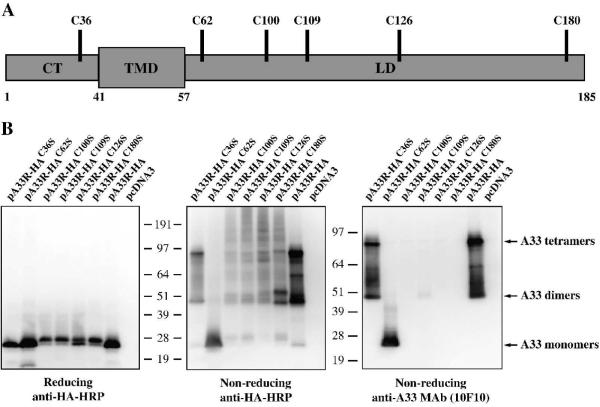
Fig. 1. Cysteine at amino acid 62 is involved in intermolecular disulfide bond formation.
(A) Schematic representation of A33. A33 is a type II transmembrane protein. The predicted cytoplasmis tail (CT), transmembrane domain (TMD), and lumenal domain (LD) are shown. The positions of the six cysteine residues are indicated. (B) Western blot analysis. HeLa cells were infected with vTF7.3 in the presence of AraC and transfected with the indicated plasmids. 24 h PI, cells were harvested and lysed. Cell lysates were resolved by SDS-PAGE under either reducing or non-reducing conditions. The proteins were transferred to nitrocellulose. The membranes were probed with an HRP-conjugated anti-HA antibody or anti-A33 MAb (10F10). The positions and molecular weights, in kDa, of marker proteins are shown.