Abstract
Background and aims
The transcription factor GATA4 is expressed throughout most of the small intestine except distal ileum, and restricts expression of the apical sodium-dependent bile acid transporter (ASBT), the rate-limiting intestinal bile acid transporter, to distal ileum. The hypothesis was tested that reduction of GATA4 activity in mouse small intestine results in an induction of bile acid transport in proximal small intestine sufficient to restore bile acid absorption and homeostasis after ileocaecal resection (ICR).
Methods
Bile acid homeostasis was characterised in non-surgical, sham or ICR mice using two recombinant Gata4 models in which Asbt expression is induced to different levels.
Results
Reduction of intestinal GATA4 activity resulted in an induction of ASBT expression, bile acid absorption and expression of bile acid-responsive genes in proximal small intestine, and a reduction of luminal bile acids in distal small intestine. While faecal bile acid excretion and bile acid pool size remained unchanged, the bile acid pool became more hydrophilic due to a relative increase in tauro-β-muricholate absorption. Furthermore, proximal induction of Asbt in both Gata4 mutant models corrected ICR-associated bile acid malabsorption, reversing the decrease in bile acid pool size and increase in faecal bile acid excretion and hepatic cholesterol 7α-hydroxylase expression.
Conclusions
Reduction of intestinal GATA4 activity induces bile acid absorption in proximal small intestine without inducing major changes in bile acid homeostasis. This induction is sufficient to correct bile acid malabsorption caused by ICR in mice.
Ileal diseases and resections result in bile acid malabsorption due to loss of intestinal bile acid transport capacity.1–3 Bile acid malabsorption is associated with diarrhoea, steatorrhoea, fat-soluble vitamin deficiencies,2 hyperoxaluria and kidney stones,4 and gallstone complications,5 and may be a risk factor for developing colorectal cancer6–8 and osteoporosis.9 Current treatments generally focus on sequestering luminal bile acids to relieve bile acid-induced diarrhoea10 or bile acid replacement treatment to reverse the steatorrhoea and improve nutrient absorption.11–13 However, these treatments do not correct bile acid malabsorption, and have only met with limited success.
Recently, we14 and others15 demonstrated that GATA4, a member of the GATA family of transcription factors that is expressed throughout most of the small intestine except distal ileum, plays an important role in maintaining jejunal–ileal differences in absorptive enterocyte gene expression. Conditional, inducible deletion of the activation domains encoded by Gata4 (ie, synthesis of GATA4Δex2),14 or conditional deletion of Gata4,15 transforms the expression of specific absorptive enterocyte genes from a jejunal to an ileal pattern. One specific change is the induction in the jejunum of the expression of the apical sodium-dependent bile acid transporter (ASBT), the ileal-specific, rate-limiting transporter for bile acid absorption. Based on this finding, we hypothesised that intestinal GATA4 is necessary for limiting Asbt gene expression and bile acid absorption to distal ileum, and that an induction of Asbt gene expression and bile acid absorption in proximal small intestine by conditionally mutating or deleting Gata4 is sufficient to correct bile acid malabsorption resulting from ileocaecal resection (ICR).
METHODS
Mice
Previously, we established a conditional, inducible Gata4 mutation model14 in which GATA4 activity in the small intestine is reduced but not eliminated (Model 1, G4Δex2, supplementary figure S1 online). To inducibly delete Gata4 in the mature small intestine, we established a new model (Model 2, G4ap, supplementary figure S1) using a previously published modification of the Gata4 allele.16 In both models, conditional recombination was targeted to the small intestinal epithelium by the VillinCreERT2 transgene.14, 17, 18 To induce recombination, and also to control for potential tamoxifen effects, all animals were treated with tamoxifen (Sigma-Aldrich, St Louis, Missouri, USA) as described.14, 18 Tissue was collected 14–17 days after the last tamoxifen injection. All mice were previously backcrossed into the C57BL/6 genetic background and only adult (6–12 week of age) males were used for study. Mice were fed ad libitum standard rodent chow containing ~5% (w/w) fibre, 60% carbohydrate, 28% protein and 12% fat (% of calories), unless indicated otherwise. Genotypes were confirmed by reverse transcriptase–PCR (RT–PCR) using previously validated primers (Supplementary figure S2 online).14, 16, 17, 19, 20 The genotypes for all control and test mice are indicated in Supplementary table S1. Approval was obtained from the Institutional Animal Care and Use Committee.
RNA isolation and RT–PCR
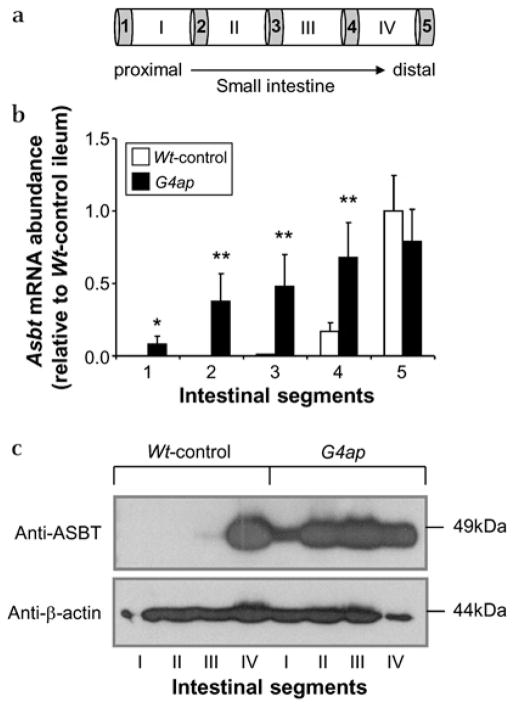
Mice were dissected and RNA was isolated from liver and small intestine as described previously.14 For RNA isolation from small intestine, the location of the tissue samples (~1.0 cm of small intestine) are depicted as grey boxes in figure 1a and correspond to five equidistant regions along the length of the small intestine. The small intestinal tissue samples designated 3 and 5 that were used for RNA isolation are also referred to as jejunum and ileum, respectively. mRNA abundances were determined by semi-quantitative and real-time RT–PCR14 using validated primer pairs (Supplementary figures S2 and S3). Glyceraldehyde-3-phosphate dehydrogenase mRNA abundance was measured for each sample and used to normalise the data.
Figure 1.
Intestinal Gata4 deletion results in an induction of apical sodium-dependent bile acid transporter (ASBT) expression in proximal small intestine. (a) Schematic representation of intestinal sampling. The grey bars numbered 1–5 indicate the ~1.0 cm segments used for RNA isolation. The white bars with Roman numerals I –IV indicate the ~7 cm segments used for the isolation of brush border membrane vesicles. (b) Real-time reverse transcriptase–PCR reveals a proximal induction of Asbt mRNA in G4ap mice. *p<0.05, **p<0.01, as compared with Wt-controls, n=4 in each group. Values are presented relative to the mean value of the Wt-control segment 5 samples. (c) Western analysis shows a proximal induction of ASBT protein in G4ap mice. β-Actin was used as a loading control.
Protein extracts and immunoblotting
After intestinal samples were obtained for RNA isolation, brush border membrane vesicles (BBMVs) were prepared as described by Kessler et al21 using tissue samples I–IV, which correspond to the four quartiles of small intestine (depicted as white boxes in figure 1a). Western analysis was conducted22 using 30 μg of BBMVs. Primary antibodies included rabbit anti-ASBT23 (1:3750) and mouse anti-β-actin (1:4000, Sigma).
Bile acid measurements
Mucosal to serosal transfer of radiolabelled taurocholate (TC) was measured in quartiles I–IV of small intestine (depicted as white boxes in figure 1a) using everted gut sacs as previously described.24 Bile acid concentrations in stools from 72 h collections and also in segmental luminal contents were determined as previously described.24–26 Bile acid pool size and composition was determined from liver, gall bladder and small intestine as previously described.24 In selected experiments, bile acid composition was also determined in luminal contents. The bile acid hydrophobicity index was calculated according to Heuman.27
Surgery
Male mice (10–12 weeks old were transferred to a liquid rodent diet (Bio-serve, Frenchtown, New Jersey, USA) 2 days prior to surgery. Mice were weighed and sham or ICR surgery was performed as previously described28 (Supplementary figure S4). In mice that underwent ICR, ~10 cm of ileum proximal to the ileocaecal junction and caecum were removed, and the colon was anastomosed to the remaining small intestine. Asbt mRNA abundance in the most proximal 1 cm sample of the resected small intestine was low or undetectable, indicating that all of the native Asbt-expressing tissue was removed. In mice that underwent sham operations, transection and anastomosis occurred ~10 cm proximal to the ileocaecal junction. Mice were given 2 ml of intraperitoneal zosyn (100 mg/kg, Wyeth Pharmaceuticals, Philadelphia, Pennsylvania, USA) in phosphate-buffered saline and the abdomen was closed. The mice were incubated at 27°C for 2–4 h after surgery, and then transferred to their normal housing, with access to liquid diet. After a week of recovery, the mice were transitioned to solid food. Three weeks after surgery, mice were treated with tamoxifen and tissue was collected as described above. All mice treated with tamoxifen were fully recovered from surgery as indicated by normal activity, weight gain to presurgical body weights, and formed stools. The overall mortality rate was 59%; virtually all mice that did not recover from surgery died of obstruction.
Statistical analyses
Data are expressed as mean±SD. Statistically significant differences were determined by the two-tailed Student t test or analysis of variance followed by the Tukey–Kramer multiple comparison test. Differences were considered statistically significant at p<0.05.
Expanded methods
More detailed description of all methodology is provided in a supplementary online only file ‘Expanded Methods Section’.
RESULTS
Intestinal Gata4 deletion results in a proximal induction of bile acid absorption
Conditional deletion of Gata4 in our new G4ap model (Model 2, Supplementary figure S1) was virtually complete, as indicated by the elimination of Gata4 mRNA and GATA4 protein, (Supplementary figure 5). Body weights (BWs), plasma triglycerides and plasma cholesterol were not different between Wt-control and G4ap mice (Supplementary table S2). Asbt mRNA (figure 1b) and protein (figure 1c), normally restricted to the distal ileum, were significantly induced throughout the small intestine of G4ap mice, suggesting that the proximal small intestine acquires the underlying capacity to absorb bile acids.
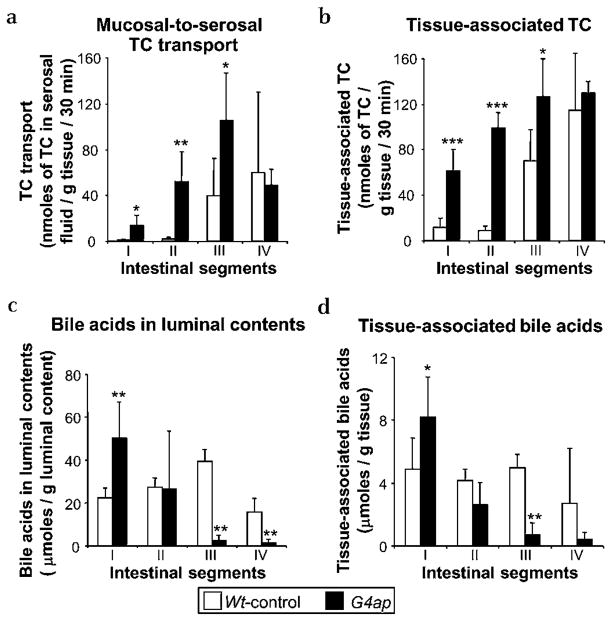
TC transport to the serosal fluid (figure 2a) and tissue-associated TC (figure 2b) in everted gut sacs were both significantly increased in proximal segments after intestinal Gata4 deletion, demonstrating directly that this region acquires the capacity to take up and transport bile acids. Bile acid excretion from a 3 day stool collection was not significantly different between Wt-control and G4ap mice (9.6±3.6 vs 12.3±1.1 μmol day−1 100 g−1 BW, n=5 in each group), indicating that intestinal Gata4 deletion does not induce major changes in overall bile acid absorption in vivo. Bile acid concentrations in luminal contents (figure 2c) and tissue (figure 2d) in Wt-control mice were similar among all four segments of small intestine, consistent with minimal bile acid absorption throughout most of the small intestine. In contrast, luminal and tissue-associated bile acids in G4ap mice were high proximally and decreased distally. The increased levels of bile acids in the luminal contents of proximal intestine after Gata4 deletion may be due to decreased intestinal motility. Inactivation of Gata4 results in increased proximal expression of peptide YY,14, 15 the ileum-derived peptide hormone that is partially responsible for mediating the ‘ileal brake’, the inhibitory feedback mechanism that slows transit of a meal through the gastrointestinal tract.29 The nearly complete depletion of bile acids in distal small intestine is consistent with an induction of bile acid absorption in the proximal small intestine.
Figure 2.
Intestinal Gata4 deletion results in an induction of taurocholate (TC) uptake in proximal small intestine, and a depletion of luminal bile acids in distal small intestine. Ex vivo measurements of TC transport in everted gut sacs show (a) a significant increase in mucosal-to-serosal transport of radioactively labelled TC in proximal intestine of G4ap mice as compared with Wt-controls, and (b) a corresponding increase in tissue-associated TC. *p<0.05, **p<0.01, ***p<0.001, as compared with Wt-controls, n=5 in each group. In vivo segmental analysis reveals that the amount of bile acid in luminal contents (c) and tissue (d) is reduced in distal small intestine of G4ap mice as compared with Wt-controls. *p<0.05, **p<0.01, as compared with Wt-controls, n=6 for G4ap mice, n=5 for Wt-control mice.
Intestinal Gata4 deletion results in altered patterns of expression of intestinal bile acid-responsive genes
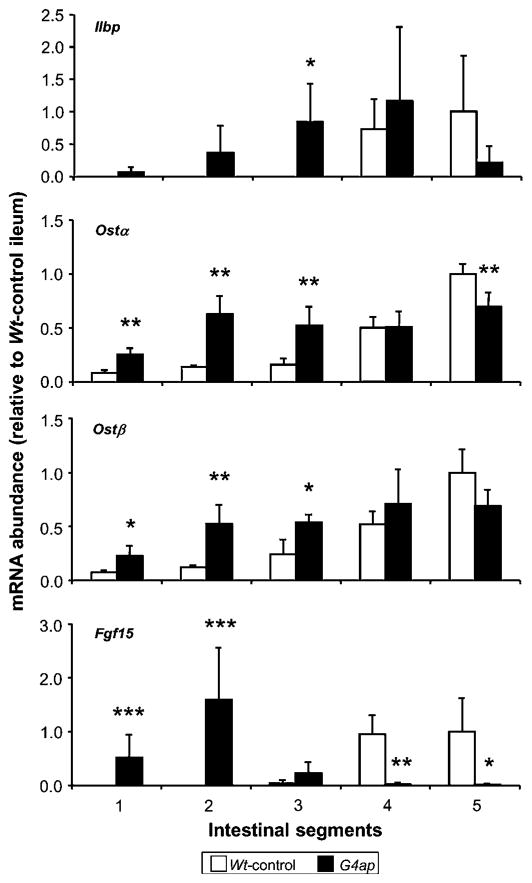
We next examined the expression of known bile acid-responsive genes, including the cytosolic bile acid-binding protein (ileal lipid-binding protein; Ilbp), subunits of the basolateral membrane bile acid transporter (organic solute transporter alpha-beta; Ostα-Ostβ), and fibroblast growth factor-15 (Fgf15), an intestine-derived regulator of hepatic bile acid synthesis. As shown in figure 3, the expression of Ilbp, Ostα, Ostβ and Fgf15 is normally most abundant in distal small intestine, but was induced (in most cases significantly) in proximal segments of small intestine after Gata4 deletion. The expression of these genes tended to be lower in distal small intestine of G4ap mice as compared with Wt-control mice, with Ostα and Fgf15 attaining statistical significance. These patterns are consistent with an increase in bile acid flux in absorptive enterocytes in proximal segments and a decrease in bile acid flux in distal segments. Fgf15 mRNA abundance was induced proximally and reduced to undetectable levels in distal segments, demonstrating a high sensitivity to bile acid flux across the absorptive enterocyte.
Figure 3.
Intestinal Gata4 deletion results in an increase in proximal and a decrease in distal expression of intestinal genes regulated by bile acids. Real-time reverse transcriptase–PCR analyses of RNA from intestinal segments along the length of small intestine show a general increase in ileal lipid-binding protein (Ilbp), organic solute transporter (Ost)α and β, and fibroblast growth factor 15 (Fgf15) mRNA abundances in proximal segments, and a decrease in distal segments of G4ap mice as compared with Wt-controls. *p<0.05, **p<0.01, ***p<0.001, as compared with Wt-controls, n=4 in each group. Values are presented relative to the mean value of the Wt-control segment 5 samples.
Intestinal Gata4 recombination results in tauro-β-muricholate (TBMC) enrichment of the bile acid pool
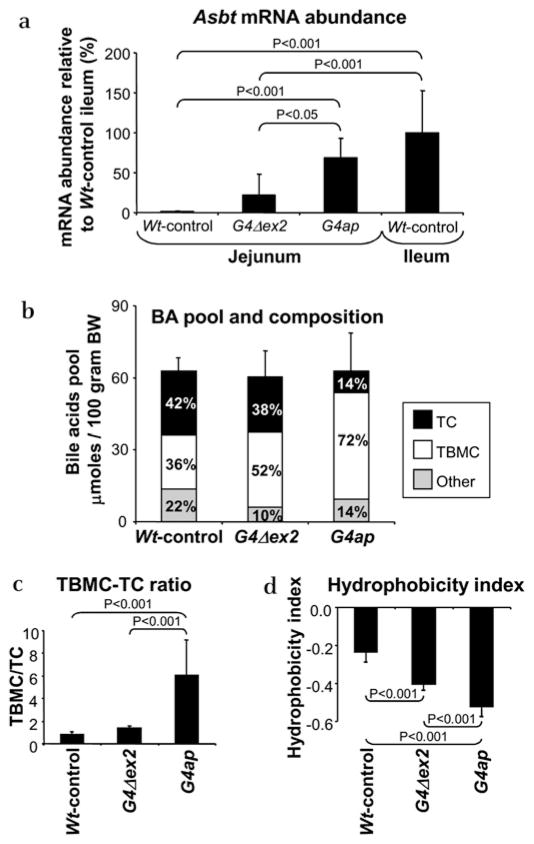
As shown in figure 4a, deletion of the activation domains of Gata4 in our previously characterised model (G4Δex2)14 resulted in an induction of Asbt mRNA in jejunum that is 22% of Wt-control ileum levels (low Asbt induction), whereas deletion of Gata4 in our new model (G4ap) resulted in an induction that is 69% of Wt-control ileum levels (high Asbt induction). The graded response of these two mouse lines was also confirmed for lactase-phlorizin hydrolase (Lph) (Supplementary figure S6), an activation target of GATA4.14 Characterisation of both models, therefore, allows us to define the effects of low versus high proximal induction of Asbt.
Figure 4.
Intestinal Gata4 recombination results in an enrichment of tauro-β-muricholate (TBMC) and reduction of taurocholate (TC) in the bile acid pool. (a) Real-time reverse transcriptase–PCR of apical sodium-dependent bile acid transporter (Asbt) mRNA in Wt-control jejunum (segment 3) and ileum (segment 5), G4Δex2 jejunum and G4ap jejunum shows a 22% and 69% transformation to wild-type ileal levels in jejunum of G4Δex2 and G4ap mice, respectively. n=6–10 in each group. (b) Bile acid (BA) pool size, as determined by the total bile acid content in liver, gall bladder and small intestine, is similar among Wt-control, G4Δex2 and G4ap mice, but demonstrates an increase in TBMC, and decrease in TC and other bile acids in G4Δex2 and G4ap mice as compared with Wt-controls. (c) The TBMC/TC ratio of the bile acid pool reveals a 2.2-fold increase in G4Δex2 mice and a 7.2-fold (p<0.001) increase in G4ap mice as compared with Wt-controls. (d) The hydrophobicity index of the bile acid pool reveals a 1.7-fold (p<0.001) decrease in G4Δex2 mice and a 2.2-fold (p<0.001) decrease in G4ap mice as compared with Wt-controls. (n=4–7 in each group).
As shown in figure 4b, the bile acid pool size did not differ among Wt-control, G4Δex2 and G4ap mice, indicating that induced bile acid absorption in the proximal small intestine does not alter the size of the bile acid pool. Surprisingly, however, the bile acid pool composition became progressively enriched in TBMC in G4Δex2 and G4ap mice (figure 4b), resulting in a significant increase in the TBMC/TC ratio (figure 4c). Because TBMC is more hydrophilic than TC,27 the hydrophobicity index of the bile acid pool was significantly decreased in G4Δex2 and G4ap mice (figure 4d).
TBMC enrichment of the bile acid pool after intestinal Gata4 deletion is due to a relative increase in TBMC uptake by the small intestine
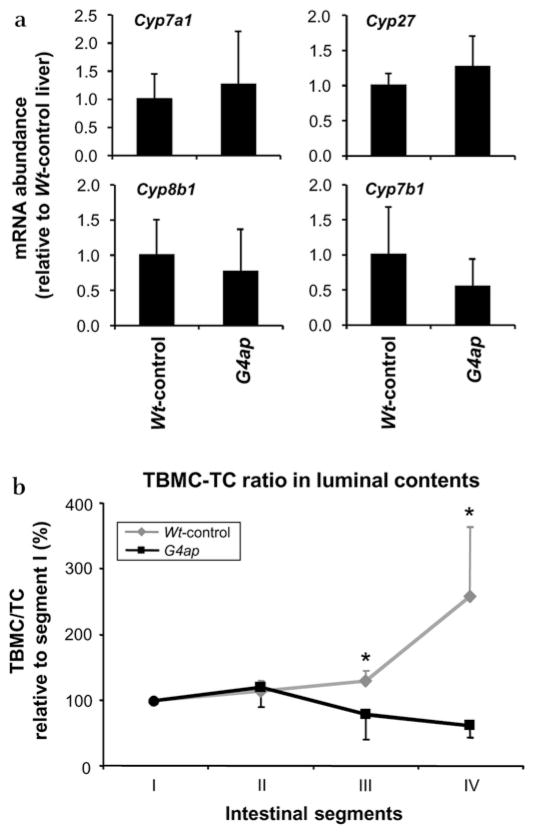
To understand the mechanism underlying the change in pool composition, we characterised potential alterations is bile acid biosynthesis in Wt-control and G4ap mice. Surprisingly, none of the mRNAs for key enzymes in the hepatic bile acid biosynthetic pathways (cholesterol 7α-hydroxylase (CYP7a1),30, 31 sterol 27-hydroxylase (CYP27),32 sterol 12α-hydroxylase (CYP8b1)33 and oxysterol 7α-hydroxylase (CYP7b1)34) were significantly different in the G4ap mice as compared with the Wt-controls (figure 5a), suggesting that the shift in bile acid composition is not due to alterations in bile acid synthesis.
Figure 5.
Tauro-β-muricholate (TBMC) enrichment of the bile acid pool after intestinal Gata4 deletion is due to an increase in TBMC uptake. (a) Real-time reverse transcriptase–PCR shows that the mRNAs for hepatic bile acid biosynthetic enzymes, Cyp7a1, Cyp8b1, Cyp27 and Cyp7b1, are similar between Wt-control and G4ap mice (n=5 in each group). Values are presented relative to the mean value of Wt-control liver samples. (b) Segmental analysis of the bile acid composition of luminal contents reveals that the TMBC/taurocholate (TC) ratio as a percentage of segment I increases distally in Wt-controls, but decreases distally in the G4ap mice. *p<0.05 as compared with Wt-controls, n=3–6 in each group.
Since studies of the bile acid substrate specificity of ASBT revealed a marked preference for TC as compared with the taurine-conjugated forms of α, β or ω-muricholic acid,35 we hypothesised that TC but not TBMC absorption is almost complete from terminal ileum in the wild-type situation, and that the shift in the bile acid pool composition is determined by enhanced absorption of TBMC as a result of increased total intestinal ASBT expression. To test this hypothesis, we assessed the TBMC/TC ratio in luminal contents along the length of the small intestine of Wt-control and G4ap mice. The TBMC/TC ratio in segment I was 3.4-fold higher in G4ap mice (2.68±1.07) as compared with Wt-controls (0.84±0.39) (p<0.05), demonstrating that the composition of the bile acids secreted into the duodenum by the gall bladder is already TBMC enriched. As shown in figure 5b, the TBMC/TC ratio in the luminal contents of Wt-control mice progressively increased distally, approaching a threefold rise from segment I, II or III to segment IV, reflecting a more efficient absorption of TC than TBMC in this limited region of distal small intestine where ASBT is expressed. In contrast, the TBMC/TC ratio in the luminal contents of G4ap mice decreased distally, and was significantly lower than that in Wt-controls in the distal half (segments III and IV, figure 5b), revealing that when ASBT is expressed over the total length of the small intestine, a greater proportion of TBMC is absorbed in the G4ap mice as compared with Wt-controls.
Intestinal Gata4 recombination corrects bile acid malabsorption associated with ICR
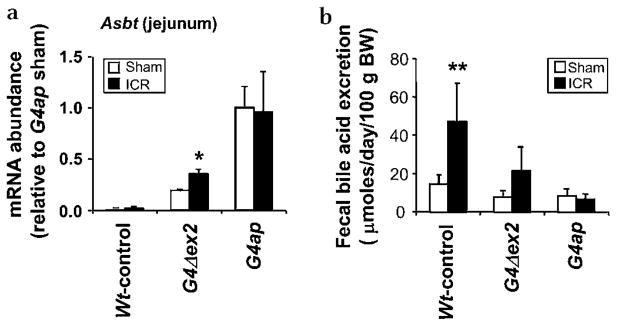
To determine if proximal induction of bile acid absorption compensates for bile acid malabsorption due to a loss of ileal function, sham or ICR surgery was performed. As shown in figure 6a, Asbt mRNA was incrementally induced in G4Δex2 and G4ap jejunum (see Supplementary figure S4 for sampling location) of both sham and ICR mice, with an increase similar to that found in non-operated animals of the respective genotypes (see figure 4a), demonstrating that sham or ICR surgery does not affect the proximal induction of Asbt. Faecal bile acid excretion in Wt-control ICR mice was significantly higher than in sham-operated mice (3.2-fold, p<0.01) (figure 6b), with six of seven excreting >25 μmol day−1 100 g−1 BW, confirming malabsorption of bile acids.1, 36, 37 In G4Δex2 ICR mice, bile acid excretion was higher than in sham-operated mice (2.8-fold, p=0.11), but only one of four excreted >25 μmol day−1 100 g−1 BW. Faecal bile acid excretion in G4ap ICR mice was similar to that in sham-operated mice, and no excretion value (n=6) was >25 μmol day−1 100 g−1 BW. These data demonstrate that low (G4Δex2) or high (G4ap) Asbt induction results in a partial or complete rescue of bile acid absorption, respectively.
Figure 6.
Intestinal Gata4 recombination restores bile acid absorption after ileocaecal resection (ICR). (a) Real-time reverse transcriptase–PCR on RNA obtained from jejunum shows an induction of the apical sodium-dependent bile acid transporter (Asbt) mRNA in sham-operated and ICR G4Δex2 and G4ap mice. Values are presented relative to the mean value of the sham-operated G4ap samples. *p<0.05 as compared with sham-operated mice, n=3–7 in each group. (b) Faecal bile acid content is increased in Wt-control ICR mice as compared with their sham-operated counterparts, but approached or returned to normal levels in G4Δex2 and G4ap mice that underwent ICR. **p<0.01 as compared with sham-operated mice, n=3–7 in each group.
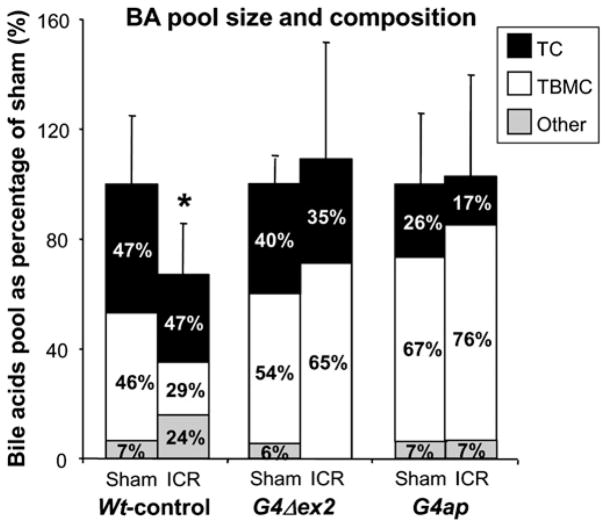
As shown in figure 7, the whole body bile acid pool size was 33% lower in Wt-control ICR mice as compared with sham-operated mice, reflecting bile acid malabsorption and an inability of de novo hepatic synthesis to compensate for bile acid loss. In contrast, the bile acid body pool was not decreased by ICR in G4Δex2 and G4ap mice as compared with their sham counterparts, suggesting that the proximal induction of bile acid absorption in both Gata4 mutant models is sufficient to maintain an efficient enterohepatic circulation of bile acids. It is noteworthy that in Wt-control mice, ICR resulted in an increase in the proportion of TC plus TC metabolite (taurodeoxycholate) and a relative decrease in TBMC in the bile acid pool. This alteration in composition is consistent with an ICR-induced increase in hepatic synthesis of TC via the CYP7A1/CYP8B1 pathway and increased TC spillage into the colon, where it is converted to deoxycholate (DC) by the bacterial flora and partially reabsorbed.38, 39 No increase of DC was found in G4Δex2 and G4ap mice after ICR, suggesting that TC is being absorbed in the proximal small intestine, before encountering the bacterial flora in the distal small intestine and colon.
Figure 7.
Intestinal Gata4 recombination prevents a loss of bile acids in the body pool after ICR. Bile acid (BA) pool size, as determined by the total bile acid content in liver, gall bladder and small intestine, is lower in Wt-control mice that underwent ileocaecal resection (ICR) as compared with sham-operated mice. Bile acid pool size in G4Δex2 and G4ap mice that underwent ICR remains similar to that of their sham-operated counterparts. *p<0.05, as compared with sham-operated mice, n=3–7 in each group.
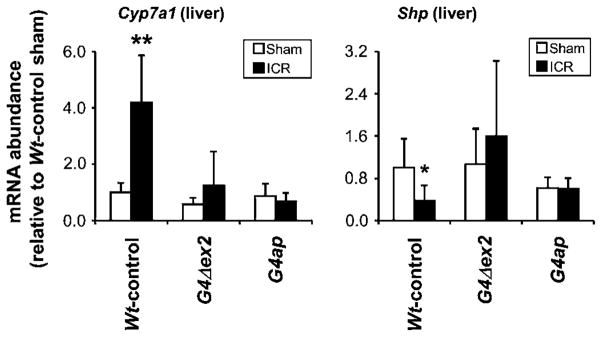
In Wt-controls, ICR resulted in a 4.2-fold increase in Cyp7a1 mRNA (p<0.01) and a 2.7-fold decrease in small heterodimer partner (Shp) mRNA (p<0.05) in liver as compared with sham-operated mice (figure 8), consistent with a compensatory upregulation of bile acid synthesis in response to bile acid malabsorption.40 Neither an increase in Cyp7a1 mRNA nor a decrease in Shp mRNA was found in G4ap mice after ICR, suggesting that proximal bile acid uptake in Gata4 mutant mice is sufficient to maintain bile acid homeostasis.
Figure 8.
Intestinal Gata4 recombination eliminates the need for compensatory upregulation in bile acid synthesis after ileocaecal resection (ICR). Real-time reverse transcriptase–PCR shows an increase in Cyp7a1 and a decrease in Shp mRNA abundance in liver of Wt-control mice that underwent ICR as compared with sham-operated mice, whereas no major differences were found between sham-operated and ICR G4Δex2 and G4ap mice. *p<0.05, **p<0.01, as compared with sham-operated mice, n=3–7 in each group. Values are presented relative to the mean value of the sham-operated Wt-control liver samples.
DISCUSSION
GATA4, a member of the evolutionarily conserved GATA transcription factor family, exhibits critical and diverse functions in cellular proliferation, differentiation and gene regulation in multiple organs.41 Using an in vivo model in which the activation domains of Gata4 are deleted, we were the first to show that GATA4 determines key differences in absorptive enterocyte gene expression between jejunum and ileum.14 Here, we show that intestinal Gata4 deletion results in an induction of Asbt gene expression and bile acid absorption in the proximal small intestine that is sufficient to restore bile acid absorption, bile acid pool size and hepatic Cyp7a1 mRNA abundance to physiological levels after ICR, without major defects in bile acid homeostasis.
Induction of intestinal Asbt expression and bile acid absorption has been previously reported. Treatment with glucocorticoid hormones precociously induces ASBT and ILBP expression in the distal small intestine during development,42–44 but the effect on proximal small intestine was not reported. Compensatory changes in ASBT expression have been shown in adult rats after ileal resection or transposition,40, 45, 46 but these changes occur only in regions of the small intestine that natively express ASBT, and are not sufficient to correct bile acid malabsorption after ileoectomy.40 Transplantation of ileal neonatal rat stem cells into adult rat jejunum leads to expression of ASBTand bile acid absorption in this segment, and reduces bile acid excretion to physiological levels after ileoectomy,47 demonstrating that ileal stem cells maintain their ileal character, and thus probably give rise to enterocytes that do not express GATA4. Our study is the first to demonstrate an induction of ASBT expression and bile acid absorption in enterocytes that normally do not exhibit these characteristics.
While absorption of bile acids in proximal small intestine does not result in alterations in faecal bile acid excretion, overall pool size (figure 4a), BW and plasma triglyceride or cholesterol levels (Supplementary table S2), our data show that proximal absorption of bile acids results in TBMC enrichment of the bile acid pool (figure 4b). An increase in the TBMC fraction of the bile acid pool was also found in Cyp8b133 and liver receptor homologue-148, 49 knockout mice. In both models, CYP8b1 is eliminated or dramatically reduced, leading to an increase in muricholic acid synthesis, and accumulation of TBMC in the bile acid pool. In our model, the TBMC enrichment of the pool was not due to alterations in the expression of hepatic bile acid biosynthetic enzymes (figure 5a), but resulted from the cumulative increase in TBMC uptake by the small intestine relative to TC (figure 5b). ASBT has a higher affinity for TC than TBMC,35 and as such the absorption of TC but not TBMC is almost complete from terminal ileum in the wild-type situation (figure 5b). By inducing ASBT expression in the proximal small intestine, the opportunity for TBMC uptake is increased, leading to a cumulative increase in the TBMC fraction in the pool over many enterohepatic cycles. Accordingly, our data demonstrate that the restriction of ASBT and active bile acid absorption to the distal small intestine by GATA4 plays a key role in determining the composition of the bile acid pool.
Using ICR in mice28 to induce bile acid malabsorption, we show that resection of terminal ileum and caecum leads to a marked increase of faecal bile acid excretion (figure 6b), a reduction of the bile acid pool (figure 7) and a compensatory upregulation of the expression of hepatic Cyp7a1 mRNA (figure 8), hallmarks of bile acid malabsorption reported in humans1 and rats40 after ileoectomy. We show here that low or high induction of Asbt expression by modulating intestinal GATA4 activity induces bile acid absorption in proximal small intestine that is sufficient to reduce bile acid excretion (figure 6B), maintain the bile acid pool size (figure 7) and reduce the compensatory upregulation of bile acid synthesis (figure 8) after ICR. This is the first example of a non-transplant intervention able to restore intestinal bile acid absorptive function following ileoectomy.
Battle et al 15 showed that conditional Gata4 deletion in the mouse small intestine results in cholesterol and fat malabsorption, and attributed this to a decrease in the expression of specific jejunal genes that encode proteins involved in these processes. It is also possible that depletion of luminal bile acids by the proximal induction of Asbt (figure 2C) results in inefficient solubilisation and absorption of lipids and cholesterol. Further, since bile acid feeding experiments have shown that TBMC causes a decrease in cholesterol absorption in the small intestine of mice,50 TBMC enrichment of the bile acid pool could contribute to reduced cholesterol absorption. Thus, the underlying cause of reduced fat and cholesterol absorption in these models remains to be determined.
In patients with mild to moderate ileal disease or limited ileal resection, bile acid malabsorption leads to diarrhoea due to increased concentrations of bile acids in the colon.51 With extensive ileal disease or resection, maldigestion of fat is caused by a decrease in bile acid secretion into the small intestine due to a reduced bile acid pool.1 Current treatments focus on the symptoms associated with bile acid malabsorption, but do not correct the bile acid malabsorption itself. Our data show that a reduction of intestinal GATA4 activity induces bile acid absorption in proximal small intestine that is sufficient to restore bile acid absorption and maintain the bile acid pool after ICR in mice. Although the effect of the proximal absorption of bile acids on fat and protein digestion and absorption, the intestinal flora and gene regulation,52 as well as the effect of inducing ileal function and reducing jejunal function by decreasing GATA4 activity remain to be determined, inducing ASBT expression in the proximal small intestine by reducing GATA4 activity may be useful in the development of therapeutic interventions for patients with bile acid malabsorption due to ileal disease or resection. An approach in which Gata4 is only partially inactivated, as in our G4Δex2 model, or by disrupting interactions between GATA4 and friend of GATA (FOG), which results in a modest jejunal induction of Asbt expression,18 might be desirable. In these situations, ASBT may be induced in the proximal small intestine to levels that restore bile acid absorption and pool size to homeostasis after ICR, but minimise potential negative side effects that may arise from the complete inactivation of Gata4.
Significance of this study.
What is already known about this subject?
Ileal diseases and resections result in bile acid malabsorption due to loss of the ileal-specific apical sodium-dependent bile acid transporter (ASBT).
Therapeutic options for restoring bile acid absorption are limited since it has not been possible to induce ASBT expression in proximal intestinal regions that do not natively express ASBT.
Reducing the activity of the transcription factor GATA4 in the small intestine induces the expression of ASBT in the jejunum.
What are the new findings?
Intestine-specific inhibition of GATA4 activity induces expression of factors required for physiologically significant bile acid absorption in proximal small intestine.
The bile acid homeostatic machinery is able to adequately accommodate bile acid absorption from the proximal small intestine following intestine-specific inhibition of GATA4 activity.
Intestine-specific inhibition of GATA4 activity results in an induction of bile acid absorption in proximal small intestine that corrects the bile acid malabsorption associated with ileocaecal resection in mice.
How might it impact on clinical practice in the foreseeable future?
Strategies to reduce or modulate intestinal GATA4 activity may be useful for restoring ileal-specific functions such as bile acid absorption in patients with ileal disease or ileal resection.
Supplementary Material
Acknowledgments
The authors thank Dr A Rao for assistance with the everted gut sac experiments, Drs R J Grand and J B Watkins for critical reading of the manuscript, and Dr M A Helmrath for guidance with the ileocaecal resections, and K A Stapleton for technical support.
Funding This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK-061382 (SDK) and R01-DK-047987 (PAD), a Harvard Digestive Disease Center grant 5P30-DK-34854, the Nutricia Research Foundation (EB), the Foundation De Drie Lichten (EB) and the Foundation Doctor Catharine van Tussenbroek (EB) in The Netherlands.
Footnotes
Supplementary figures, tables and methods are published online only. To view these files please visit the journal online (http://gut.bmj.com).
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Hofmann AF. Bile acid malabsorption caused by ileal resection. Arch Intern Med. 1972;130:597–605. [PubMed] [Google Scholar]
- 2.Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr. 2001;32:407–17. doi: 10.1097/00005176-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113:1767–78. doi: 10.1053/gast.1997.v113.pm9352883. [DOI] [PubMed] [Google Scholar]
- 4.Smith LH, Fromm H, Hofmann AF. Acquired hyperoxaluria, nephrolithiasis, and intestinal disease. Description of a syndrome. N Engl J Med. 1972;286:1371–5. doi: 10.1056/NEJM197206292862601. [DOI] [PubMed] [Google Scholar]
- 5.Pitt HA, Lewinski MA, Muller EL, et al. Ileal resection-induced gallstones: altered bilirubin or cholesterol metabolism? Surgery. 1984;96:154–62. [PubMed] [Google Scholar]
- 6.Kanamoto R, Azuma N, Suda H, et al. Elimination of Na+-dependent bile acid transporter from small intestine by ileum resection increases [correction of increase] colonic tumorigenesis in the rat fed deoxycholic acid. Cancer Lett. 1999;145:115–20. doi: 10.1016/s0304-3835(99)00240-2. [DOI] [PubMed] [Google Scholar]
- 7.Morvay K, Szentleleki K, Torok G, et al. Effect of small bowel resection on fecal bile acid excretion and on experimental colon tumour in rats. Acta Chir Hung. 1990;31:25–31. [PubMed] [Google Scholar]
- 8.Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A:1067–70. doi: 10.1016/0959-8049(95)00216-6. [DOI] [PubMed] [Google Scholar]
- 9.van Hogezand RA, Banffer D, Zwinderman AH, et al. Ileum resection is the most predictive factor for osteoporosis in patients with Crohn’s disease. Osteoporos Int. 2006;17:535–42. doi: 10.1007/s00198-005-0016-7. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–58. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 11.Emmett M, Guirl MJ, Santa Ana CA, et al. Conjugated bile acid replacement therapy reduces urinary oxalate excretion in short bowel syndrome. Am J Kidney Dis. 2003;41:230–7. doi: 10.1053/ajkd.2003.50012. [DOI] [PubMed] [Google Scholar]
- 12.Gruy-Kapral C, Little KH, Fordtran JS, et al. Conjugated bile acid replacement therapy for short-bowel syndrome. Gastroenterology. 1999;116:15–21. doi: 10.1016/s0016-5085(99)70223-4. [DOI] [PubMed] [Google Scholar]
- 13.Kapral C, Wewalka F, Praxmarer V, et al. Conjugated bile acid replacement therapy in short bowel syndrome patients with a residual colon. Z Gastroenterol. 2004;42:583–9. doi: 10.1055/s-2004-813059. [DOI] [PubMed] [Google Scholar]
- 14.Bosse T, Piaseckyj CM, Burghard E, et al. Gata4 is essential for the maintenance of jejunal–ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–70. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battle MA, Bondow BJ, Iverson MA, et al. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–86.e1. doi: 10.1053/j.gastro.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B, Ma Q, Rajagopal S, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el Marjou F, Janssen KP, Chang BH, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–93. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 18.Beuling E, Bosse T, aan de Kerk DJ, et al. GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Dev Biol. 2008;322:179–89. doi: 10.1016/j.ydbio.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pu WT, Ishiwata T, Juraszek AL, et al. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275:235–44. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Kessler M, Acuto O, Storelli C, et al. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978;506:136–54. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- 22.van Wering HM, Bosse T, Musters A, et al. Complex regulation of the lactase-phlorizin hydrolase promoter by GATA-4. Am J Physiol Gastrointest Liver Physiol. 2004;287:G899–909. doi: 10.1152/ajpgi.00150.2004. [DOI] [PubMed] [Google Scholar]
- 23.Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–34. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- 24.Rao A, Haywood J, Craddock AL, et al. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci USA. 2008;105:3891–6. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson PA, Haywood J, Craddock AL, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–7. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- 26.Repa JJ, Dietschy JM, Turley SD. Inhibition of cholesterol absorption by SCH 58053 in the mouse is not mediated via changes in the expression of mRNA for ABCA1, ABCG5, or ABCG8 in the enterocyte. J Lipid Res. 2002;43:1864–74. doi: 10.1194/jlr.m200144-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Heuman DM. Quantitative estimation of the hydrophilic–hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–30. [PubMed] [Google Scholar]
- 28.Dekaney CM, Fong JJ, Rigby RJ, et al. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1013–22. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 29.Van Citters G, Lin HC. The ileal brake: a fifteen year progress report. Curr Gastroenterol Rep. 1999;1:404–9. doi: 10.1007/s11894-999-0022-6. [DOI] [PubMed] [Google Scholar]
- 30.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–51. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 32.Rosen H, Reshef A, Maeda N, et al. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J Biol Chem. 1998;273:14805–12. doi: 10.1074/jbc.273.24.14805. [DOI] [PubMed] [Google Scholar]
- 33.Li-Hawkins J, Gafvels M, Olin M, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li-Hawkins J, Lund EG, Turley SD, et al. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol Chem. 2000;275:16536–42. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- 35.Kramer W, Stengelin S, Baringhaus KH, et al. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604–17. [PubMed] [Google Scholar]
- 36.Tougaard L, Giese B, Pedersen BH, et al. Bile acid metabolism in patients with Crohn’s disease in terminal ileum. Scand J Gastroenterol. 1986;21:627–33. doi: 10.3109/00365528609003110. [DOI] [PubMed] [Google Scholar]
- 37.Hardison WG, Rosenberg IH. Bile-salt deficiency in the steatorrhea following resection of the ileum and proximal colon. N Engl J Med. 1967;277:337–42. doi: 10.1056/NEJM196708172770704. [DOI] [PubMed] [Google Scholar]
- 38.Wells JE, Berr F, Thomas LA, et al. Isolation and characterization of cholic acid 7alpha-dehydroxylating fecal bacteria from cholesterol gallstone patients. J Hepatol. 2000;32:4–10. doi: 10.1016/s0168-8278(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 39.Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66:1107–13. doi: 10.1128/aem.66.3.1107-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Ansari N, Xu G, Kollman-Bauerly K, et al. Analysis of the effect of intestinal resection on rat ileal bile acid transporter expression and on bile acid and cholesterol homeostasis. Pediatr Res. 2002;52:286–91. doi: 10.1203/00006450-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–52. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 42.Hwang ST, Henning SJ. Hormonal regulation of expression of ileal bile acid binding protein in suckling rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1555–63. doi: 10.1152/ajpregu.2000.278.6.R1555. [DOI] [PubMed] [Google Scholar]
- 43.Hwang ST, Henning SJ. Ontogenic regulation of components of ileal bile acid absorption. Exp Biol Med (Maywood) 2001;226:674–80. doi: 10.1177/153537020222600713. [DOI] [PubMed] [Google Scholar]
- 44.Barnard JA, Ghishan FK. Methylprednisolone accelerates the ontogeny of sodium-taurocholate cotransport in rat ileal brush border membranes. J Lab Clin Med. 1986;108:549–55. [PubMed] [Google Scholar]
- 45.Coppola CP, Gosche JR, Arrese M, et al. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection in the rat. Gastroenterology. 1998;115:1172–8. doi: 10.1016/s0016-5085(98)70088-5. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya T, Kalogeris TJ, Tso P. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am J Physiol. 1996;271:G681–91. doi: 10.1152/ajpgi.1996.271.4.G681. [DOI] [PubMed] [Google Scholar]
- 47.Avansino JR, Chen DC, Hoagland VD, et al. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg. 2005;201:710–20. doi: 10.1016/j.jamcollsurg.2005.06.270. [DOI] [PubMed] [Google Scholar]
- 48.Lee YK, Schmidt DR, Cummins CL, et al. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22:1345–56. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mataki C, Magnier BC, Houten SM, et al. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27:8330–9. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang DQ, Tazuma S, Cohen DE, et al. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285:G494–502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62:918–34. [PubMed] [Google Scholar]
- 52.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–83. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.