Abstract
Mouse NIH 3T3 cells expressing the human c-fms protooncogene encoding the receptor for colony-stimulating factor 1 (CSF-1) are able to proliferate in serum-free medium containing platelet-derived growth factor (PDGF), insulin, transferrin, and albumin as the only exogenous proteins. When PDGF and insulin were replaced by purified human recombinant CSF-1, the cells became spindle shaped and refractile, were no longer contact inhibited, and proliferated to high densities. Thus, transduction of the human c-fms gene into mouse fibroblasts can not only reprogram their growth factor requirements but can also induce ligand-dependent features of cell transformation. NIH 3T3 cells stably transformed by the feline v-fms oncogene or by a mutated, oncogenic human c-fms gene were able to proliferate in the absence of exogenous growth factors. A monoclonal antibody that prevents signal transduction by the human CSF-1 receptor inhibited the growth of cells transformed by the activated c-fms oncogene, confirming that CSF-1 receptor function was required to abrogate growth factor requirements and to maintain the transformed state.
Full text
PDF
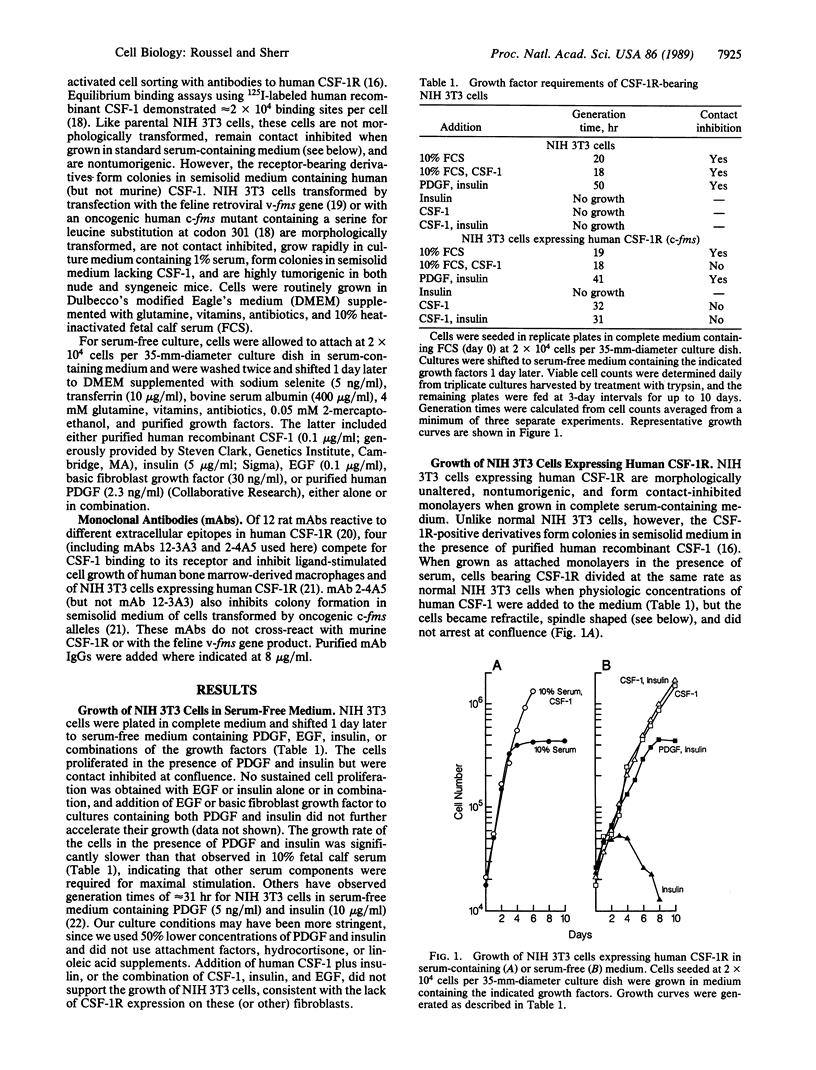


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmun R. A., Look A. T., Roberts W. M., Roussel M. F., Seremetis S., Ohtsuka M., Sherr C. J. Monoclonal antibodies to the human CSF-1 receptor (c-fms proto-oncogene product) detect epitopes on normal mononuclear phagocytes and on human myeloid leukemic blast cells. Blood. 1989 Feb 15;73(3):827–837. [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Lyman S. D., Park L., Rohrschneider L. R. Colony stimulating factor-1 induced growth stimulation of v-fms transformed fibroblasts. Oncogene. 1988 Oct;3(4):391–395. [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlofsky A., Stanley E. R. CSF-1-induced gene expression in macrophages: dissociation from the mitogenic response. EMBO J. 1987 Oct;6(10):2947–2952. doi: 10.1002/j.1460-2075.1987.tb02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Ashmun R. A., Rettenmier C. W., Sherr C. J. Colony-stimulating factor 1-mediated regulation of a chimeric c-fms/v-fms receptor containing the v-fms-encoded tyrosine kinase domain. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5903–5907. doi: 10.1073/pnas.85.16.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Ashmun R. A., Downing J. R., Ohtsuka M., Quan S. G., Golde D. W., Roussel M. F. Inhibition of colony-stimulating factor-1 activity by monoclonal antibodies to the human CSF-1 receptor. Blood. 1989 May 15;73(7):1786–1793. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol. 1985 Feb;122(2):221–228. doi: 10.1002/jcp.1041220210. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Woolford J., McAuliffe A., Rohrschneider L. R. Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell. 1988 Dec 23;55(6):965–977. doi: 10.1016/0092-8674(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Zhan X., Goldfarb M. Growth factor requirements of oncogene-transformed NIH 3T3 and BALB/c 3T3 cells cultured in defined media. Mol Cell Biol. 1986 Oct;6(10):3541–3544. doi: 10.1128/mcb.6.10.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]