Abstract
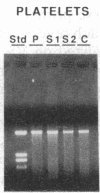
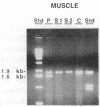
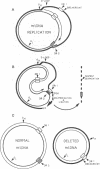
The muscle mitochondria of a patient with Kearns-Sayre/chronic external ophthalmoplegia plus syndrome were found to be completely deficient in respiratory complex I activity and partially deficient in complex IV and V activities. Treatment of the patient with coenzyme Q10 and succinate resulted in clinical improvement of respiratory function, consistent with the respiratory deficiencies. Restriction enzyme analysis of the muscle mtDNA revealed a 4.9-kilobase deletion in 50% of the mtDNA molecules. Polymerase chain reaction analysis demonstrated that the deletion was present in the patient's muscle but not in her lymphocytes or platelets. Furthermore, the deletion was not present in the muscle or platelets of two sisters. Hence, the mutation probably occurred in the patient's somatic cells. Direct sequencing of polymerase chain reaction-amplified DNA revealed a 4977-base-pair deletion removing four genes for subunits of complex I, one gene for complex IV, two genes for complex V, and five genes for tRNAs, which paralleled the respiratory enzymes affected in the disease. A 13-base-pair direct repeat was observed upstream from both breakpoints. Relative to the direction of heavy-strand replication, the first repeat was retained and the second repeat was deleted, suggesting a slip-replication mechanism. Sequence analysis of the human mtDNA revealed many direct repeats of 10 base pairs or greater, indicating that this mechanism could account for other reported deletions. We postulate that the prevalence of direct repeats in the mtDNA is a consequence of the guanine-cytosine bias of the heavy and light strands.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Boursot P., Yonekawa H., Bonhomme F. Heteroplasmy in mice with deletion of a large coding region of mitochondrial DNA. Mol Biol Evol. 1987 Jan;4(1):46–55. doi: 10.1093/oxfordjournals.molbev.a040421. [DOI] [PubMed] [Google Scholar]
- Bresolin N., Bet L., Binda A., Moggio M., Comi G., Nador F., Ferrante C., Carenzi A., Scarlato G. Clinical and biochemical correlations in mitochondrial myopathies treated with coenzyme Q10. Neurology. 1988 Jun;38(6):892–899. doi: 10.1212/wnl.38.6.892. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Stroynowski I., Wallace D. C. Characterization of mitochondrial DNA in chloramphenicol-resistant interspecific hybrids and a cybrid. Somatic Cell Genet. 1980 Jul;6(4):543–554. doi: 10.1007/BF01539155. [DOI] [PubMed] [Google Scholar]
- Goda S., Hamada T., Ishimoto S., Kobayashi T., Goto I., Kuroiwa Y. Clinical improvement after administration of coenzyme Q10 in a patient with mitochondrial encephalomyopathy. J Neurol. 1987 Jan;234(1):62–63. doi: 10.1007/BF00314013. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Cooper J. M., Morgan-Hughes J. A., Harding A. E. Deletions of muscle mitochondrial DNA. Lancet. 1988 Jun 25;1(8600):1462–1462. doi: 10.1016/s0140-6736(88)92273-8. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns D. R., Drachman D. B., Hurko O. Identical mitochondrial DNA deletion in blood and muscle. Lancet. 1989 Feb 18;1(8634):393–394. doi: 10.1016/s0140-6736(89)91779-0. [DOI] [PubMed] [Google Scholar]
- Landi L., Cabrini L., Sechi A. M., Pasquali P. Antioxidative effect of ubiquinones on mitochondrial membranes. Biochem J. 1984 Sep 1;222(2):463–466. doi: 10.1042/bj2220463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestienne P., Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet. 1988 Apr 16;1(8590):885–885. doi: 10.1016/s0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Moore A. R., Grossman L. I. The frequency of matching sequences in DNA. J Theor Biol. 1984 May 7;108(1):111–122. doi: 10.1016/s0022-5193(84)80172-1. [DOI] [PubMed] [Google Scholar]
- Ogasahara S., Nishikawa Y., Yorifuji S., Soga F., Nakamura Y., Takahashi M., Hashimoto S., Kono N., Tarui S. Treatment of Kearns-Sayre syndrome with coenzyme Q10. Neurology. 1986 Jan;36(1):45–53. doi: 10.1212/wnl.36.1.45. [DOI] [PubMed] [Google Scholar]
- Ogasahara S., Yorifuji S., Nishikawa Y., Takahashi M., Wada K., Hazama T., Nakamura Y., Hashimoto S., Kono N., Tarui S. Improvement of abnormal pyruvate metabolism and cardiac conduction defect with coenzyme Q10 in Kearns-Sayre syndrome. Neurology. 1985 Mar;35(3):372–377. doi: 10.1212/wnl.35.3.372. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Yoneda M., Tanaka M., Ohno K., Sato W., Suzuki H., Nishikimi M., Yamamoto M., Nonaka I., Horai S. Maternal inheritance of deleted mitochondrial DNA in a family with mitochondrial myopathy. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1240–1247. doi: 10.1016/0006-291x(88)90272-0. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Rotig A., Colonna M., Bonnefont J. P., Blanche S., Fischer A., Saudubray J. M., Munnich A. Mitochondrial DNA deletion in Pearson's marrow/pancreas syndrome. Lancet. 1989 Apr 22;1(8643):902–903. doi: 10.1016/s0140-6736(89)92897-3. [DOI] [PubMed] [Google Scholar]
- Rötig A., Colonna M., Blanche S., Fischer A., Le Deist F., Frezal J., Saudubray J. M., Munnich A. Deletion of blood mitochondrial DNA in pancytopenia. Lancet. 1988 Sep 3;2(8610):567–568. doi: 10.1016/s0140-6736(88)92687-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Zheng X. X., Lott M. T., Shoffner J. M., Hodge J. A., Kelley R. I., Epstein C. M., Hopkins L. C. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988 Nov 18;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Sato T., Anno M., Ujike H., Takemoto M. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes with recurrent abdominal symptoms and coenzyme Q10 administration. J Neurol Neurosurg Psychiatry. 1987 Nov;50(11):1475–1481. doi: 10.1136/jnnp.50.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]