Abstract
Phenology refers to the periodic appearance of life-cycle events and currently receives abundant attention as the effects of global change on phenology are so apparent. Phenology as a discipline observes these events and relates their annual variation to variation in climate. But phenology is also studied in other disciplines, each with their own perspective. Evolutionary ecologists study variation in seasonal timing and its fitness consequences, whereas chronobiologists emphasize the periodic nature of life-cycle stages and their underlying timing programmes (e.g. circannual rhythms). The (neuro-) endocrine processes underlying these life-cycle events are studied by physiologists and need to be linked to genes that are explored by molecular geneticists. In order to fully understand variation in phenology, we need to integrate these different perspectives, in particular by combining evolutionary and mechanistic approaches. We use avian research to characterize different perspectives and to highlight integration that has already been achieved. Building on this work, we outline a route towards uniting the different disciplines in a single framework, which may be used to better understand and, more importantly, to forecast climate change impacts on phenology.
Keywords: phenology, seasonal timing, circannual rhythms, reproductive physiology, molecular genetics, avian reproduction
1. Introduction
Phenological records of periodically recurring life-cycle events go back thousands of years (Foster & Kreitzman 2009). One of the striking observations is the large difference in the between-year variation in phenology: some seasonal events occur so reliably, for example the return of certain migratory species, that they have been likened to ‘calendars’, whereas others show high year-to-year variation in the date at which they occur (Gwinner & Helm 2003). In the temperate zone, annual variation in phenology often correlates with environmental variables, most often with temperature. It is therefore not surprising that global climate change, including an increase in average temperatures, has led to clear shifts in phenology, but also with large differences between species (Schwartz 2003; Parmesan 2006). The variation in response to climate change between species at different trophic levels indicates that many phenological shifts currently remain inadequate and lead, for example, to uncoupling of phenological events within food chains (Visser et al. 1998; Visser & Both 2005; Memmott et al. 2007; Post & Forchhammer 2008; Miller-Rushing et al. 2010; Singer & Parmesan 2010). Mistiming has consequences at the population level (Nussey et al. 2005; Both et al. 2006). Thus, phenology is a key process that may link climate change to population persistence and possibly to community composition (Miller-Rushing et al. 2010).
In order to assess the ecological consequences of climate change it is essential to forecast phenology under different scenarios, such as provided by the Intergovernmental Panel for Climate Change (IPCC). This forecasting is hampered by two major problems (Visser 2008): phenology needs to be forecasted for environments well outside the range of natural conditions observed by phenologists. Predictions must therefore rely on additional information, in particular about the causal (mechanistic) basis of the relationship between phenology and environmental conditions. Furthermore, organisms may adapt via micro-evolution. Hence, the relationship between phenology and environment is changing over time, and this rate of adaptation needs to be incorporated in the forecasting (van Asch et al. 2007). To meet these two challenges, it is crucial that research on phenological events integrates mechanistic and evolutionary perspectives.
A variety of disciplines study the seasonality of plant and animal life-cycle events. These include researchers who consider themselves phenologists but also ecologists, who would term it seasonal timing, and chronobiologists, who focus on timing programmes and on underlying mechanisms such as circannual rhythms. Similarly, physiologists, who are studying reproduction or any other seasonal life-cycle stages, and more recently molecular ecologists who look at the genetic make up of individuals, are also interested in within-season variation (e.g. Wilczek et al. 2010). It is obvious that while all these disciplines deal with the same phenomenon, they take different angles to it and aim at different endpoints. This is due to historical differences, as they approach phenology after having developed in diverse contexts. Over recent decades, all disciplines have made remarkable progress in unravelling detailed information underlying phenology and although there have been a number of excellent examples of integration (see §3), we believe this is the time to promote further integration of different disciplines.
In this paper, we will highlight the insights and the limitations of the diverse approaches to phenology, and we outline a route towards uniting the different disciplines in a single framework. We will use this framework to provide an outlook to what kind of research is needed to forecast phenology influenced by climate change. As the common basis of our combined backgrounds, we use avian timing as an example of how integration could be achieved.
2. Different perspectives
Different approaches to phenology focus on different aspects of seasonal phenomena. Researchers in the field of phenology observe in a standardized way periodic plant and animal life-cycle events over long periods of time, sometimes using phenological stations (Menzel & Fabian 1999; Schwartz 2003) and relate the inter-annual variations to climatic variables. Thus, they approach seasonal recurrence as a phenomenon in its own right, with a clear interest in year-to-year variation, but mainly in the first individuals or (less often) the population mean rather than the variation among all individuals of a population. Phenologists are well aware of (climate) changes in the long run and can provide a comprehensive picture of modified timing on a level of local populations and communities, often in a wide range of species and over large geographical regions.
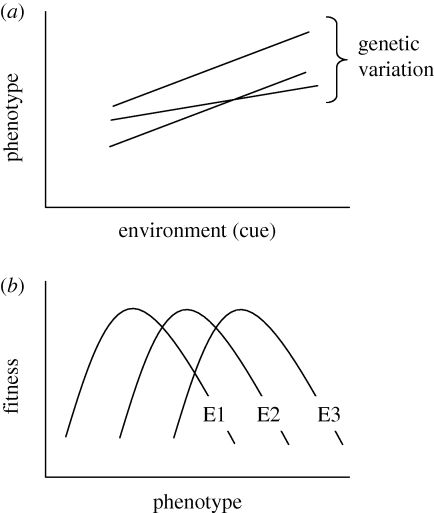
In contrast, population-level data are less central for other disciplines studying phenology. Phenological events, such as flowering date, return date of birds and egg laying date of sea turtles, can also be studied as characteristics of individuals within populations. In this approach, the phenological event is seen as the phenotype of a given individual. As a step towards integrating disciplines, studies of phenological trait values as phenotypes can be framed in the concept of phenotypic plasticity (Pigliucci 2005). If an individual is phenotypically plastic, its phenotype is shaped by the interaction between the genotype and the environment. Different genotypes in the same environment will give rise to different phenotypes, while the same genotype will lead to different phenotypes in different environments (figure 1a). The curve describing the relationship between phenotype and environmental variables is termed the reaction norm. While traditionally phenotypic plasticity was used for morphological traits shaped during ontogeny, it is now also widely used for traits that are expressed multiple times in an organism's life (Nussey et al. 2005), such as phenological events. An example would be the lay date of an individual bird in different spring environments over consecutive years.
Figure 1.
(a) A phenological trait value, the phenotype, can be shaped by the environment: the same genotype gives rise to different phenotypes in different environments (the trait is phenotypically plastic). Different genotypes have different reaction norms: their phenotypes are affected differently by the environment; environmental factors on the x-axis represent those that are used as predictive cues for phenology. (b) Different phenotypes have different fitness depending on the environment (E1–E3). Note that the environment in (a) is often a different environment than the environment of selection in (b) (see text).
Below, we use the conceptual background of phenotypic plasticity to highlight differences between disciplines and to characterize their particular approaches to explain a specific phenological event in a given year, focusing on avian reproduction.
(a). The evolutionary ecologist's view
Evolutionary ecologists refer to phenological events as seasonal timing, and put emphasis on the variation among individuals within years as well as on the between-year variation. Year-to-year variation should ultimately be explained by corresponding year-to-year variation in the seasonality of the environment (Baker 1938; Visser et al. 2004). For example, timing of reproduction in small forest passerine birds of the temperate zone is affected by temperature because the time of the peak abundance of the nestlings’ food is correlated with this temperature (Visser et al. 2006). Well-timed breeding is thought to confer benefits from higher fitness both in terms of enhanced offspring survival and possibly increased condition of the parents to survive until the next breeding season (Thomas et al. 2001; figure 1b). Thus, in an evolutionary ecologist's view, birds have been selected for their ability to have their chicks in the nest at the time of peak food abundance.
However, timely reproduction requires anticipation of suitable conditions well in advance. A bird has to make a ‘decision’ whether to initiate preparations for breeding long before its chicks will be exposed to the environmental conditions that determine reproductive success. Thus, environmental variables at the time of ‘decision-making’ are often used as predictive cues and thereby also function as proximate (mechanistic) causes that influence reproductive timing (Baker 1938). Because the use of cues is crucial for optimal timing, an evolutionary ecologist wants to know whether responsiveness to environmental information (cues) has evolved to correctly predict the time to initiate breeding. An evolutionary ecologist is not primarily interested in the causal mechanism but is very much aware what the cues should provide: they should predict the future environment under which the phenotype will be selected. Key characteristics of this future environment of selection include conspecific and multi-trophic interactions. Thus, evolutionary ecologists try to find the cues that are reliably linked to these characteristics. There is no a priori reason why just a single environmental variable should act as a cue, and it is a pragmatic oversimplification that evolutionary ecologists often consider phenotypic plasticity as the relationship between timing and just a single environmental variable. More generally, the reaction norm should have a multi-dimensional environmental axis (Visser 2008).
Evolutionary ecologists mainly study organisms in the wild as they are interested in the fitness benefits of different timing strategies and in selection on timing. In collaboration with quantitative geneticists they estimate the heritability of timing and the response to selection; i.e. the rate of micro-evolution. An important limitation is that extrapolation beyond the natural range, as needed for predictions under climate change, is not feasible without a more mechanistic understanding of phenology (see also §4a).
(b). The physiologist's view
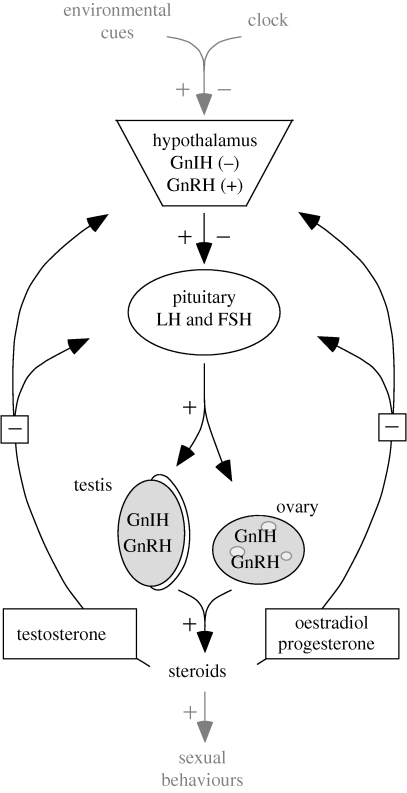
Physiologists have a clear interest in how the changes within an annual cycle causally come about. They ask how organisms use environmental cues to adjust changes in morphology, physiology and behaviour over the course of their annual schedule, often with a strong emphasis on the effects of photoperiod (the annual change in day length) on the orchestration of successive life-history stages (i.e. progression of events; Farner et al. 1966; Follett et al. 1985; Wingfield 2008). The physiological mechanisms underlying avian timing of reproduction, one of the most explicit examples of phenology, describe how cues are perceived and transduced at the level of the hypothalamo–pituitary–gonadal (HPG) axis (figure 2). These environmental cues have been classified in several different groups (Wingfield & Kenagy 1991). The most important ones for seasonal breeding are probably the initial predictive cues, which allow preparations well ahead of breeding (i.e. photoperiod), and the supplementary and social cues (i.e. temperature, rainfall, interactions with conspecifics, food abundance), which allow fine-tuning of timing to local, and year-specific, conditions.
Figure 2.
A classical view of the hypothalamo–pituitary–gonadal (HPG) axis (in black) and its integration with phenology (in grey): the relevant environmental cues (e.g. photoperiod) interact with the permissive clock mechanisms to stimulate (plus sign) or inhibit (minus sign) the secretion of the gonadotrophin-inhibitory (GnIH) and -releasing hormones (GnRH) by the hypothalamus into the portal veins, which in turn interact to regulate the release of the gonadotrophins (luteinizing hormone, LH, and follicle-stimulating hormone, FSH) by the pituitary into the general circulation. LH and FSH bind to receptors in the ovary and testis, stimulate their development, the gametogenesis and their production of steroid hormones (mainly testosterone in males, oestradiol and progesterone in females). These steroids are involved in other physiological and morphological changes (e.g. secondary sexual characters) and increase the probability of several sexual behaviours, such as courtship and egg-laying, occurring. They also act through negative feedback mechanisms on the higher levels of the HPG axis. Note that GnIH and GnRH have recently been identified in the gonads as well, where they potentially act as regulators (see review in Ubuka et al. 2008).
Photoperiodism has been massively studied and the effects of photoperiod on the HPG axis are well described (figure 2; see reviews in Farner 1985; Follett et al. 1985; Dawson et al. 2001; Sharp 2005), while our knowledge of the effects of the supplementary and social cues is patchy. Most insights have been achieved on the effect of temperature as a cue (Wada et al. 1990; Silverin & Viebke 1994; Wingfield et al. 1996, 1997, 2003; Maney et al. 1999; Meijer et al. 1999; Perfito et al. 2005; Salvante et al. 2007; Silverin et al. 2008; Visser et al. 2009) but there is a serious lack of understanding on how temperature is integrated at the level of the HPG axis (see §4b). Similarly, some studies have shown marked effects of other supplemental cues, such as food and water availability, and social cues (Moore 1983; Vleck & Priedkalns 1985; Hahn et al. 1995; Zann et al. 1995; Hau et al. 2000; O'Brien & Hau 2005; Helm et al. 2006; Small et al. 2007; Voigt et al. 2007; Perfito et al. 2008). Most of these studies were carried out on a behavioural level, but some are now addressing links to the HPG axis (e.g. effects of social cues; Moore 1983; Stevenson et al. 2008).
Physiologists are in general less interested in year-to-year variation or variation among individuals (Ball & Balthazart 2008; Williams 2008; but see Wingfield et al. 1992) than in detailed, typically experimental, studies of individuals. In an environmental context, physiologists study between-species (or between-populations) variation within an annual cycle with an emphasis on average values. Classically, physiology often requires careful measurements under controlled conditions, and hence much of the work is done in the laboratory and restricted to a specific mechanism. This has major advantages and physiologists have made important contributions to our knowledge of the detailed molecular and genetic basis of the physiological system. When related to phenology of free-living animals there are, however, a few shortcomings (Calisi & Bentley 2009). For example, the environmental variables used in an experimental set-up usually are set to arbitrarily chosen values (often well outside the natural range) and often these variables are kept constant under experimental conditions (e.g. 20°C throughout the day and night, 16 h of light for several consecutive weeks).
A disadvantage specific for work on phenology of avian reproduction is the use of male rather than female animals (Ball & Ketterson 2008) while it is probably that females are more important in determining seasonal timing (Caro et al. 2009). Partly this is because under captive conditions males commonly develop fully active reproductive conditions, while females often do not reach full breeding status, especially when caged individually (but see Calisi & Bentley 2009). As a consequence most studies do not describe the links between physiological mechanisms and the complex interactions that determine lay dates in the wild. Physiological studies commonly study gonadal (generally testis) development to examine reproductive cycles. This allows a detailed understanding of different phases in the breeding cycle and of their respective regulatory mechanisms. However, the value of gonadal cycles as proxies of lay dates may be limited, and there have been few attempts to validate these proxies against actual field data (Helm 2009; Visser et al. 2009). Gonadal development is, however, also studied by physiologists as a seasonal process in itself, rather than as a proxy for actual laying dates.
(c). The chronobiologist's view
Chronobiologists study daily and annual fluctuations in physiology and behaviour and focus on internal timing programmes that enable organisms to cope with, and anticipate, geophysical cycles in the environment. Fundamental for this field is the observation that periodic events on a daily or annual scale often persist endogenously, i.e. in complete absence of external time information given by Zeitgebers (timing cues) like light and darkness. Such endogenous daily (‘circadian’) or annual (‘circannual’) rhythms continue with period lengths that differ slightly from 24 h or 365 days, respectively (Gwinner 1986, 2003; Kumar et al. 2004; Bradshaw & Holzapfel 2007; Paul et al. 2008; Helm 2009). Thus, without any seasonal cues, many animals can maintain annual cycles of moult, migration, gonadal development, pupation or hibernation for many years (Gwinner 1986, 2003; Nisimura & Numata 2001; Kondo et al. 2006) by solely relying on changes of their circannual clock. Zeitgebers synchronize rhythms by determining their period (i.e. the length of a cycle) and their phase (i.e. the time when a particular fraction of the cycle occurs), and additionally, other factors may modify (‘mask’) the expression of rhythms. However, species differ greatly in the strength of the underlying circannual clock and in requirements for the environmental input (Dawson et al. 2001; Goldman et al. 2004; Bradshaw & Holzapfel 2007; Paul et al. 2008; Helm 2009).
When chronobiologists look at phenology, they focus on the annual cycle and variation over a year, but are less interested in variation between years and individuals. The chronobiologist's view on why phenological events occur and when they occur emphasizes the interplay between internal time-structuring and environmental cues. The seasonal clock determines how sensitive an animal is to external cues (Helm et al. 2009). These cues will for some phases of the seasonal clock lead to stimulation of the system, while at other phases they will have little or no impact (see below).
The chain of steps from cue input to the specific output, i.e. laying date, is still poorly understood, but it involves a calendar-and-clock system. The much better known circadian clock entrains to the 24 h light cycle and provides a ‘reference clock’ for the reading of calendrical information (i.e. photoperiod; Sharp 2005). The calendrical information that is thereby attained is modulated by a bird's internal (circannual) calendar, e.g. interpreting a 12 h day as ‘long’ or ‘short’, respectively in a seasonal context (Helm et al. 2009). If a bird is in the correct phase for photostimulation, a cascade of gene expression starts, and the genes involved in this cascade are now being rapidly revealed (Ono et al. 2009). Subsequently, in different brain regions, different aspects of physiology and behaviour are activated that further prepare reproduction (e.g. growth of song nuclei, melatonin receptor density change; Bentley & Ball 2000). The activation and development of these processes then leads, via physiological and behavioural feedback loops, to specific responsiveness to relevant environmental factors (nutritional levels, social stimuli, etc.). Thereby, the interplay between clock and local environment determines the precise timing of the phenological event, i.e. the seasonal phenotype.
A limitation of this approach is a focus on experimental, and often constant, conditions (Calisi & Bentley 2009). Thus, despite the stated interest of chronobiologists in understanding temporal behaviour, relatively little is known about how the underlying calendar-and-clock system relates to ‘timing in the real world’ (Menaker 2006; Wikelski et al. 2008). However, comparative studies of related taxa indicate that timing programmes are tailored to particular life histories, and an evolutionary angle is starting to develop (Gwinner 1986; Bradshaw & Holzapfel 2007; Helm et al. 2009).
(d). The molecular geneticist's view
Molecular geneticists approach phenological events from two sides: on one hand, they aim at understanding the genetic variation among individuals in the timing of the event and the selection on this variation. On the other hand, they aim at understanding the causal mechanism underlying the effect of the environment on the event. Several approaches can be used to identify genes that are involved in the variation among individuals (the genotypes for phenological events; Tauber & Kyriacou 2005). The polymorphisms can be found either by a candidate gene approach, where orthologues of (mainly mammalian) clock genes are cloned (candidate gene approach; e.g. Chong et al. 2000; Yoshimura et al. 2000; Fidler & Gwinner 2003), or by a genome-wide approach, where random markers are used to identify the variation in genome regions (polymorphisms) that are associated with the between-individual variation in a trait like the timing of a phenological event (quantitative trait locus (QTL) approach; e.g. Leder et al. (2006)).
In birds, only one candidate gene has been investigated in an ecological or evolutionary seasonal context: clock. Repeat length variations are reported to vary between species (Fidler & Gwinner 2003), and between populations (Johnsen et al. 2007) and within natural populations (Liedvogel et al. 2009). Johnsen et al. (2007) showed that in blue tits, a latitudinal cline exists in mean repeat length, with a higher mean repeat number at higher latitudes. They hypothesize that these population differences may be caused by adaptation to the variation in photoperiodic parameters between the populations (Johnsen et al. 2007). This was tested on a within-population level by Liedvogel et al. (2009), who found that females with shorter mean repeat lengths had earlier lay dates. Whereas ecological studies using candidate genes are rare, to the best of our knowledge, studies that investigate the adaptive significance of gene expression profiles are completely absent in wild birds.
Investigation of genes involved in a causal pathway is done by exploring the variation in transcription, translation and post-translational expression level (through e.g. mRNA, microarrays, proteomics) of clock genes. The expression of these genes has been localized in several brain areas, including the avian pineal gland, putative suprachiasmatic nuclei (SCN), retina and hypothalamus (Yasuo et al. 2003; Kumar et al. 2004). The expression levels give insight into the physiological processes that are up- or downregulated, causing rhythmic phenotypic expression of a trait. For this purpose, most genetic information on seasonal timing comes from research done on circadian rhythms.
The two molecular genetic approaches to phenological events (i.e. a focus on genomic variation and a focus on expression of causal pathways) are likely to identify common, but also unique sets of genes involved in regulation of heritable quantitative traits (e.g. Le Mignon et al. 2009). For instance, there may be no genomic variation in some of the genes that play a crucial role in the pathway. These can therefore not account for genetic variation among individuals and thus cannot be picked up using a QTL approach (e.g. Zou & Zeng 2009). However, they may be identified in expression studies, since the expression levels of these specific genes may be important drivers of a rhythmic expression of a trait and may be heritable by themselves (Brem & Kruglyak 2005). The differences between the approaches can be illustrated by comparison to an engine that can be set by a switch and is thereafter driven by a converter belt. Based on this analogy, microarray studies are likely to pick the genes involved in the ‘converter belt’, i.e. those genes that are involved in rhythmic seasonal fluctuations in reproductive hormones. On the other hand, QTL studies may be more likely to pick up the ‘switch genes’ that set the converter belt in motion. Physiologists, in collaboration with molecular geneticists, therefore play an important role in the investigation of both the expression of ‘converter belt’ genes and ‘switch genes’ (Ono et al. 2009).
A limitation in molecular genetic research on seasonal timing, and on phenological events in general, is that the genetic basis that underlies circannual rhythms is unknown. While day-length-dependent gene expression is relatively well understood, the knowledge of genes that underlie rhythmic expression over the season still needs to be developed. Another limitation is that conflicting results often arise owing to the use of different species, different photoperiods, variation in entrainment protocols, and potentially different mRNA detection techniques (Helfer et al. 2006). This makes a comparative study between species with different life histories problematic.
3. Integration towards a single framework
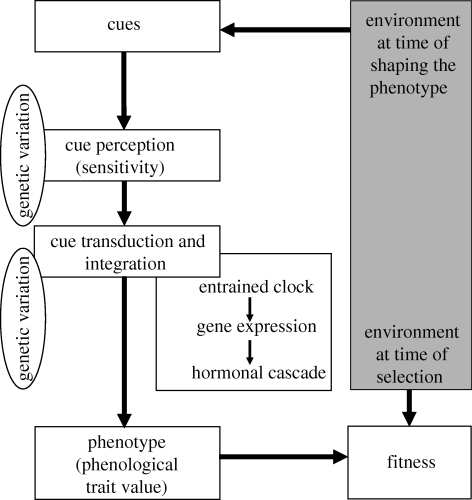
The approaches to phenology outlined above have the potential to complement each other. In figure 3, we outline a common framework that refers to all these disciplines, with the aim of a more conclusive understanding of phenology. Below, we develop this framework in further detail and emphasize promising steps towards integrating the different approaches. A first section gives a combined overview over the sequence of processes leading to a phenotypic event (in our example this is timing of avian reproduction). A second section briefly introduces a model system of integration, reproductive timing in blue tits (Cyanistes caeruleus).
Figure 3.
A unified framework for a more conclusive understanding of phenology, integrating chronobiology, physiology, molecular genetics and evolutionary ecology (see §3 of the text).
(a). An integrated view of a phenological event
The trait that is observed in phenological studies (the phenotype) will be under natural selection, and hence, the processes that are studied by all above disciplines occur in an evolutionary context. Often, relatively small differences in timing of reproduction or arrival date in migrant bird species have large fitness consequences (Nussey et al. 2005; Pulido 2007). Because fitness consequences depend on a species' ecology and the particular environment, there is no single mechanism that fits all species. Thus, a crucial step towards understanding a given phenological event involves taking account of variation between species or populations, as well as between individuals within a population.
(i). Shaping the phenotype
An animal perceives cues from its environment. Cues are environmental variables that have predictive qualities for subsequent, suitable conditions to carry out a given life-cycle event. The perception of suitable cues will affect an animal's decision to initiate preparatory steps, and hence, affects phenology (seasonal timing). Cues can be abiotic factors, like temperature, but also biotic factors, like the development of the vegetation or social cues from conspecifics. Which environmental variables actually are cues varies from species to species, and sometimes from population to population within a species. The cues used by an animal need to predict the environment of selection, i.e. the conditions under which the selection on the phenological trait takes place, in order to be useful. For example, rainfall does not predict the phenology of forest caterpillars and is thus not a cue for a great tit (Parus major) in the Netherlands, but rainfall does predict the phenology of ripe grass seeds and it is therefore a cue for zebra finches (Taeniopygia guttata) in Australia (Zann et al. 1995; Perfito et al. 2006). This kind of variation even exists within species. Different subspecies of white-crowned sparrows (Zonotrichia leucophrys) differ in whether or not they use temperature as a cue; the Arctic subspecies shows no response to temperature in their testis and follicle size development and this can be understood from the fact that the short Arctic breeding season is restricted to a period of long photoperiod, while other subspecies have to rely on supplementary cues to account for between-year variation (Wingfield et al. 1996, 1997, 2003; Maney et al. 1999). A similar pattern has been found for great tits (Silverin & Viebke 1994; Silverin et al. 2008).
The animal's sensitivity to cues varies over the year as the response mechanism is seasonal clock-dependent. Photoperiod is generally seen as the most powerful seasonal cue. Many organisms, including birds, can be induced to carry out phenological events ‘out of season’ by photoperiodic manipulation (Dawson et al. 2001; Goldman et al. 2004; Bradshaw & Holzapfel 2007), and some can be forced to undergo several annual cycles within a single year (Gwinner 1986). But even responses to photoperiod depend on the phase of the underlying, circannual cycle (Miyazaki et al. 2005; Lincoln et al. 2006; Helm et al. 2009). For example, while increasing day length stimulates breeding early in the year, even permanent light cannot re-stimulate reproduction after breeding in most species (‘photorefractoriness’; Dawson et al. 2001; Hahn & MacDougall-Shackleton 2008). In addition, there is also evidence for possible effects of temperature on circannual clocks (e.g. in hibernating mammals; Mrosovsky 1986) and for dependence of the sensitivity to temperature on the phase of the clock. For example, temperature does not influence the physiological breeding development in quails (Coturnix coturnix japonica) but a decrease in temperature, in combination with a decrease in photoperiod, is necessary to fully stop their reproductive activity (Wada et al. 1990; Wada 1993). In other species, the same temperature has a stronger effect under longer photoperiods (i.e. later in spring) than under short photoperiods (Gienapp et al. 2006; Bauer et al. 2008), and early versus late during mammalian hibernation (Wikelski et al. 2008). Similarly, when the photoperiod gets very long, it can override the lack of supplemental cues and birds can breed under very low ambient temperatures (e.g. Lambrechts et al. 1997b).
Cues from the environment are thus perceived, transduced and integrated by an animal's clock-dependent and -independent response mechanism. This leads to genes being switched on (initially in the brain), which in turn may switch on the genes involved in the pathway processes (the ‘converter belt’ genes). After these converter belt genes have been activated, a complex process with positive and negative feedback loops is started. Hormones clearly play a crucial role in this process and some pathways are well understood. The perceived environmental cues induce a cascade of neuroendocrine reactions along the HPG axis that are essential in the synchronization of the breeding cycle (figure 2). These hormones increase the probability of sexual behaviours occurring, but also play various morphological and physiological roles, including some negative feedback on higher levels of the HPG axis (Wingfield & Moore 1987; Ball & Balthazart 2002; Dawson & Sharp 2007).
While the successive levels of the HPG axis and their interactions have been well described, our knowledge on how supplementary environmental cues, other than photoperiod, are modulating the maturation of the reproductive system remains rudimentary. This is particularly true for the late stages of reproductive development in females, the rapid follicular growth that occurs in the last days before egg laying. As a consequence, the link between the activation and modulation of the HPG axis and the actual egg laying is less well understood. Under cold conditions, egg laying will be delayed in the field and perhaps temperature plays a role in this final fine-tuning stage (Meijer et al. 1999). But also unpredictable events such as severe weather conditions or territory loss will determine the laying decision and may induce temporary switching to an emergency life-history stage (Wingfield et al. 1998).
(ii). Selection on the phenotype
Animals within populations are genetically diverse. One can think of genetic variation in cue perception, transduction and integration. This genetic variation will lead to differences in the reaction norm (figure 1a). In some cases, individuals will vary in how much their phenotype is responsive to variation in cues, which will lead to variation in the slope of the reaction norm (Nussey et al. 2005). In addition, some animals will have a consistently earlier phenotype than others, and these will differ in the elevation of the reaction norm.
Teasing apart genetic variation at the perception level from genetic variation in the transduction mechanisms is a difficult task, especially in a phenological context where the cues acting on the physiology are gradually interacting and have long-lasting effects. This challenge can only be addressed if brain regions responsible for the filtering and the processing of environmental information are known. Researchers can then determine whether the expression of genetic variation occurs before or during the transduction of the signal considered as a relevant cue. In the context of seasonal reproduction, the pioneering studies by Heideman and colleagues on white-footed mice (Peromyscus leucopus; e.g. Heideman et al. 1999; Heideman & Pittman 2009) are important steps in this direction. Populations of white-footed mice differ geographically in the extent to which reproduction is inhibited under short day lengths (Heideman et al. 1999). By selective breeding and quantitative genetic analyses of variation, Heideman and co-workers showed high heritability of photoresponsiveness. Follow-up studies tracked down heritability of gonadotropin-releasing hormone (GnRH) neuron characteristics and also provided evidence for genetic variation in the nutritional and hormonal inputs to GnRH neurons (Heideman & Pittman 2009). Limited evidence exists also for plasticity and genetic variation in the perceptual mechanisms. For example, seasonal and population variations in hearing capabilities have, respectively, been demonstrated in several species of birds (Lucas et al. 2002, 2007) and in at least one species of frog (the cricket frog, Acris crepitans; Ryan et al. 1992).
Regardless of whether genetic variation affects the perception or the transduction level, variation in the way cues are ‘translated’ into a phenotype enables natural selection to act on phenotypic plasticity; i.e. on the reaction norms (Visser et al. 2004; Heideman & Pittman 2009). Thus, it is not so much the trait value itself that selection acts on but rather the physiological response mechanism underlying the phenotype. This also implies that perhaps not all the reaction norms are possible, as evolution may be constrained by the particular components of the response mechanism that show genetic variation. Furthermore, natural selection probably rarely acts on isolated physiological mechanisms, but rather on a suite of correlated traits (McGlothlin & Ketterson 2007). This is particularly true for hormones that often act simultaneously on a wide variety of characters (Ketterson & Nolan 1999), which may also constrain the response to natural selection (Lessells 2008, but see Hau 2007). Similar constraints could apply to modifications of the circadian clock system that is involved in seasonal as well as daily timing (Bradshaw & Holzapfel 2007; Heideman & Pittman 2009).
How well a phenotype performs in terms of fitness depends on the environment. For many species, the phenology of their environment, and thus of their food sources or their predators, varies from year to year. The fitness of a bird making a decision on a certain date therefore varies from year to year. It is thus a combination of the environment at the time of selection and the phenotype that determines the fitness of an individual (figure 1b).
In many cases the environmental variables that serve as cues, involved in shaping the phenotype, are not the same environmental variables that form the environment at the time of selection (Visser et al. 2004). Especially in phenology, the phenotype is often formed in a different environment from the one where selection takes place. For example, in migratory birds, the decision to depart the wintering grounds is made at a different location (environment) than the place where they are selected to be on time (their breeding grounds). This is also depicted in figure 1a,b: in figure 1a the phenotype is determined by responses to predictive environmental cues (x-axis) while in figure 1b the fitness of a given phenotype depends on the environment of selection (E1–E3). This highlights the importance of a correlation between the ‘cue’ and the ‘environment of selection’: only if the environment of selection is predicted by an environmental variable can it serve as a predictive cue.
(b). An example of interdisciplinary integration: reproductive timing in Mediterranean blue tits
In the Mediterranean region, bird populations have to cope with strong habitat heterogeneity owing to geographical variation in the vegetation structure of the landscape. Mediterranean forests consist of a mosaic of patches dominated either by broad-leaved deciduous or evergreen tree species. Blue tits successfully breed in both habitats, but have to deal with pronounced spatial variation in the phenology of their food. Deciduous forests present an early phenology, with young leaves appearing approximately one month earlier than in evergreen woods. This temporal difference in bud burst results in a similar difference in the onset in leaf-eating caterpillar outbreaks between the habitats (Zandt et al. 1990). Blue tits, like many other insectivorous bird species, depend highly on that brief peak in caterpillar abundance to raise their chicks. Differences in the timing of food abundance have important consequences for fitness and have been the subject of considerable research in the field of evolutionary ecology over the past 30 years (Blondel et al. 1993, 1999, 2006; Lambrechts et al. 1997a, 2004). In fact, the geographical and temporal variability in the availability of this food resource is one of the main selection factors driving the breeding phenology of the different blue tit populations, with high energetic and survival costs for birds failing to match their chick-rearing period with the annual short peak in caterpillar availability (Thomas et al. 2001). Since the discovery that blue tits may breed up to one month apart depending on the type of habitat in which they settle (Blondel & Isenmann 1979), these birds have been used in many studies aiming to understand how the phenology of breeding has evolved. Furthermore, research examined how this local adaptation has sometimes been constrained by the characteristics of the habitats and the birds' ability to adaptively respond to selection processes (e.g. the homogenizing effect of gene flow; see Dias 1996; Blondel et al. 2006).
Selection operates on the mechanisms underlying phenotypic plasticity and local specialization rather than on the phenotype itself (see above), and thus a significant part of the work conducted on these blue tit populations aimed to decipher the proximate organization of their breeding phenology. Furthermore, as studies of the proximate organization mainly aimed at mechanistic responses to environmental cues, a combination of field data and common garden experiments manipulating these cues was necessary (Visser & Lambrechts 1999). So, the breeding phenology of blue tits from different Mediterranean populations was also studied in captivity, first in outdoor aviaries exposed to natural environmental conditions, then under diverse combinations of natural and artificial photoperiods. Under natural settings, the laying divergence observed in the field persisted, suggesting an (at least partial) genetic determination of these population differentiations (Lambrechts & Dias 1993). Under manipulated day lengths, the breeding difference was modulated by the duration of the photoperiod, suggesting that the laying differentiation was maintained by a population divergence in the perception or transduction mechanisms to photoperiod (Lambrechts et al. 1996, 1997b; Lambrechts & Perret 2000). Subsequent work on Corsican birds combined behavioural, neurobiological and endocrinological studies in the field, and sought to describe where in the HPG axis the population differentiation resided. No difference in the early seasonal recrudescence of the hypothalamic GnRH, the testis volumes, the song production and the brain nuclei controlling song were found between the males of the populations (Caro et al. 2005a,b, 2006). In females, however, the follicle growth periods were clearly distinct between the populations, and quantitative genetic analyses of laying dates demonstrated that the optimal breeding differentiation was driven by the females (Caro et al. 2009). This demonstrated that assessing sex differences in cue perception, transduction and integration are essential components to take into account in phenological studies (Ball & Ketterson 2008), and that local adaptation to environmental heterogeneity could be a sex-limited phenomenon (Caro et al. 2009). Local population differentiations have also been demonstrated in other species, e.g. differences in (neuro-) hormones and gonadal cycles in male great tits (Silverin et al. 2008), rufous-collared sparrows (Zonotrichia capensis; Moore et al. 2005, 2006), in male and female stonechats (Saxicola torquata; Helm 2009) and between urban and forest blackbirds (Turdus merula; Partecke et al. 2004, 2005).
Altogether, the understanding of phenotypic and genetic variation in the breeding phenology of the Mediterranean blue tits has required the close integration of most disciplines interested in phenological events, including evolutionary ecology, behavioural ecology, energetics, quantitative genetic, endocrinology and neurobiology. Although some genetic differentiation in the expression of neutral genetic markers, i.e. mini- and microsatellite loci (Dias et al. 1996; Charmantier 2000), and clock gene polymorphism have been described in these blue tit populations (Johnsen et al. 2007), the possibility of a seasonal clock differentiation mechanism between populations, and the genes involved, remain to be investigated (cf. Liedvogel et al. 2009). Finally, if we want to predict adaptability to environmental variation it will also be critical to understand which environmental cues are important, how these cues are integrated in the HPG axis, and how these proximate mechanisms are inherited between generations.
4. Outlook
Understanding the mechanisms underlying a specific phenology, which is constantly under natural selection, requires the integration of disciplines studying phenology from different angles, as outlined in §3 (figure 3). Here, we discuss a number of possible research questions that address less well-understood phenological topics in the context of avian reproduction for which we think it is pressing to make substantial progress in the coming years.
(a). Adapting to changing environments
One of the most obvious impacts of climate change on nature is the shift in phenology that has been observed in many taxa (Parmesan 2006), including the laying dates in birds (Crick & Sparks 1999). This phenomenon is what we would expect for any phenological trait that is phenotypically plastic and where temperature acts as a cue. However, the question is not whether these shifts occur but rather whether these shifts are adaptive: do organisms shift their phenology the exact amount they should? This seems rarely the case (Visser & Both 2005), as in many cases the shift in phenology is either too weak or too strong to precisely match the shifts in phenology of other species in the food chain. This leads to mistiming, which may have severe fitness consequences for an individual (Visser et al. 1998; Thomas et al. 2001) as well as for population viability (Nussey et al. 2005; Both et al. 2006).
The paradox is thus that species are often phenotypically plastic in their phenology concerning temperature changes but that their response to increasing temperatures is not adaptive. This can be understood from figure 3. If climate change affects the environmental variables that serve as cues differently from the environmental variables that form the environment at the time of selection, then the response to climate change will no longer be adaptive. In other words, cues lose their predictive value. In extreme cases, the cues are not affected (birds relying on photoperiod in their overwintering area to depart to the breeding grounds) but the environment of selection is affected by climate change (the phenology of food for nestlings in the breeding area). A similar argument will also hold for resident species: climate change is unlikely to increase temperatures in a similar way throughout the year and such differential warming can cause an uncoupling of cues and the environment of selection (Visser et al. 1998): the cues perceived early on are no longer accurately predicting the future (Visser et al. 2004). Reaction norms may thus be no longer adaptive under climate change; animals have to be less or more temperature-sensitive than they are. This leads to natural selection on these reaction norms (Nussey et al. 2005), or in other words, to selection on the mechanisms underlying phenology.
For natural selection to operate on the response mechanisms underlying phenology, there needs to be genetic variation in these mechanisms (Nussey et al. 2005; Charmantier et al. 2008; Visser 2008). This makes the quest for genetic variation pressing. In many cases, physiological work is carried out on animals that are related to each other, but information on family relations is rarely used to estimate genetic resemblance, while no additional experiments would be necessary. Therefore, re-analysing existing data could be highly rewarding, provided that some pedigree data are available.
The absence of genetic variation in one or more components of the underlying mechanism could severely hamper micro-evolutionary change. In fact, at present there is very little evidence of such current micro-evolution in phenology (Gienapp et al. 2006), with perhaps a few examples such as the timing of diapause in the pitcher plant mosquito, which has shifted to longer photoperiods under a warming climate (Bradshaw & Holzapfel 2007). Ultimately, the rate of micro-evolution compared with the rate of climate change will determine the ecological impact of climate change (Visser 2008). Thus, a better understanding of the genetic variation in the response mechanism is essential.
(b). Integration of non-photic cues
In §3a, we have summarized the relatively well-understood effect of photoperiod on the mechanism underlying the phenology of avian reproduction. Effects of other environmental factors on the reproductive system have also been clearly demonstrated, in particular for social cues, food and temperature (reviewed by Lewis & Orcutt 1971; Hahn et al. 1997; Helm et al. 2006; Voigt et al. 2007; Silverin et al. 2008). However, little is known about how non-photoperiodic cues, as well as contributions from the circannual clock, are integrated to shape phenological events. Additionally, we know little about the dependence of cue integration on the state of an animal (e.g. its nutritional status), and whether reproductive status is a precondition to integrate certain cues.
The currently most comprehensive model of avian reproductive timing, developed by Sharp (2005), involves the circadian clock for daily time-measurement and two additional, interacting components. The first consists of the photoperiodically controlled GnRH-gonadotrophin and vasoactive intestinal peptide (VIP)-prolactin neuroendocrine axes. These are complemented by the neuropeptide gonadotropin-inhibitory hormone (GnIH), which is regulated by the circadian hormone melatonin, and allows negative regulation of gonadotropin synthesis and release (Tsutsui et al. 2007, 2009; Perfito & Bentley 2009). An emerging body of research describes modulation of the GnRH system in response to photoperiodic, supplemental and social cues, and thus supports its putative role as site of integration (e.g. Hahn et al. 1997; Moore et al. 2006; Ball & Ketterson 2008; Stevenson et al. 2008; Heideman & Pittman 2009; MacDougall-Shackleton et al. 2009). A second component of the model of avian reproductive timing comprises genotype-dependent, neural inputs to, or intrinsic activities of, GnRH and VIP neurons (Sharp 2005), that could contribute to variation between individuals. So far our understanding of the mechanisms that drive phenology is mostly centred on photoperiodism and hampered by the scarcity of information on other cues. One of the problems with non-photic cues, in particular temperature, is that their actions are probably slower and more progressively integrated than cues such as photoperiod, which can induce rapid responses in the brain and elsewhere that can relatively easily be measured (using microarrays, immediate-early gene expression or hormone concentrations; Meddle & Follett 1997). In addition, detection and transduction of non-photic cues is also much less well understood than that of photoperiodic cues.
Another challenge is to integrate ontogenetic effects on phenology into the physiological response mechanism. There is for instance a clear effect of photoperiod experienced by birds during rearing on their phenology of migration timing in the first year of life (Helm et al. 2005). Experiences during early adult lifetime (learning) can also affect the phenology of subsequent reproduction (Wingfield & Jacobs 1999), such as in blue tits where birds that bred too late in one year would advance their laying date in the next year (Grieco et al. 2002). Lasting ontogenetic effects, for example those related to previous experience with environmental cues (Sockman et al. 2004), may have also confounded results of various experimental studies (Calisi & Bentley 2009).
(c). The role of the seasonal clock
The sensitivity of an animal to external cues involved in phenology depends on the seasonal clock. The detailed way in which the endogenous system responds to Zeitgebers appears to have evolved such that an optimal adjustment of seasonal activities to the environment is achieved. So far, however, evolutionary and ecological aspects of synchronization have barely been studied. This is partly owing to the difficulty of maintaining and observing sufficient numbers of captive organisms over at least a year. Recent studies on carpet beetles (Anthrenus verbasci; e.g. Nisimura & Numata 2001; Miyazaki et al. 2005) have revealed in detail how the circannual cycle of an animal interacts with photoperiod. Without external information, carpet beetles pupate in circannual intervals. The number of circannual cycles required for pupation is strongly affected by the quality of larval nutrition (Miyazaki et al. 2009). If provided with day length information the beetles synchronize pupation to the external year, but the timing response depends on when in their circannual cycle the Zeitgeber information is applied. Thus, the beetles either accelerate or delay pupation as described by a so-called ‘phase response curve’ (Miyazaki et al. 2005).
In our view, a phase response curve is a chronobiological, special case of a reaction norm. This idea, i.e. that annual activities are synchronized and adaptively modulated by photoperiod, has been pursued in studies of a passerine bird, the stonechat (e.g. Gwinner 2003; Helm 2009; Helm et al. 2009). This species is equipped with a circannual clock that persists for many cycles and is normally synchronized by the annual photoperiodic cycle. Comparative studies of populations from different locations revealed strong evidence for an innate basis of geographical differences in the responsiveness of certain phases of the circannual clock to its photoperiodic Zeitgeber. These differences can best be described in terms of population specificity in the reaction norm of the circannual oscillator to photoperiod. We suggest that further studies of adaptive modifications of circannual response mechanisms are an important step towards achieving a common evolutionary framework for the study of phenology (figure 3).
The mechanisms that underlie the seasonal clock and its interactions with environmental cues are only beginning to be explored (Lincoln et al. 2006). Early ideas that seasonal clocks arise as a summation of shorter processes, for example by ‘counting’ of circadian days (Gwinner 1986; Wikelski et al. 2008), or by a fixed sequence of interdependent physiological states (Mrosovsky 1970), has not been supported. Instead, new studies point to long-term feedback processes on a tissue level (Lincoln et al. 2006) and to possible effects of energy turnover on the period length of the circannual clock, but these ideas still require further testing (Wikelski et al. 2008). The genetic basis of circannual rhythms is currently also unknown. A possible, underlying entraining mechanism could be in seasonal changes in the rhythmic expression of circadian candidate genes (e.g. Helfer et al. 2006; Martin et al. 2008; Davie et al. 2009). An example is the CRY–PER system (Cryptochrome–Period system; e.g. El Halawani et al. 2008; Hazlerigg & Loudon 2008). Expression of PER and CRY is based on a daily rhythm and locked to dawn and dusk, respectively. Therefore, changes in day length modify the interval between the expression peaks of PER and CRY and could thereby transduce information to the reproductive system (Paul et al. 2008). In spring, a shorter interval between CRY and PER may thereby contribute to entrainment of the circannual clock (Hazlerigg & Loudon 2008). However, these are speculative, early ideas about genes that may contribute to the functioning of the circannual clock (Notter & Cockett 2003; Notter 2008).
(d). Common experimental systems
Integration of approaches to phenology would be greatly accelerated if common experimental systems were developed or further expanded. All too often, ecologists experimentally study female egg-laying dates of a single wild population, physiologists study male gonadal size in the laboratory and researchers of phenology look at whole population shifts in timing on a large geographical scale. There are some examples of integrated study systems, like the white-crowned sparrow, which is studied in the wild and in the laboratory (Perfito et al. 2005), and the blue (see §3) and great tit (Drent et al. 2003). In the latter species even the same individuals are studied under controlled conditions in the laboratory as well as in the wild (Visser et al. 2009).
Setting up joined experiments is, in our view, mainly a matter of finding other researchers who are interested in a similar phenological trait but from a different perspective. Some species, like sparrows, juncos, starlings and great tits, are used by many different research groups and it would be highly feasible to do different measurements on the same individuals or at least on animals from the same population. This would be possible if researchers that need to obtain animals for their laboratory studies would be provided with nestlings from an evolutionary ecological population study, and used these hand-reared animals for detailed studies on the underlying mechanism. This would bring together the physiological and ecological knowledge of animals from a single population. Moreover, as the birds used are related, with perhaps a known pedigree, this also opens up the possibility to look at genetic variation. We strongly advocate such approaches.
A historically grown discrepancy is the persistent difference between evolutionary ecologists and physiologists in the choice of the sex they work with (females versus males) and the environmental variable settings (4 versus 20°C difference between experimental groups, or a naturally increasing photoperiod versus transfer from 8 h to 16 h of light). But we are confident that there will be enough common ground, and enough to be gained, to overcome these problems.
(e). Concluding remarks
Phenology can be studied at the level of populations, but to understand how the year-to-year variation in the phenology of populations comes about, we will need to unravel more of the phenology of individuals and understand how the underlying mechanisms have been shaped by evolution. Individuals vary their phenology from year to year in response to cues from their environment, but are restricted by the possibilities of their evolutionary history, ranging from genetic variation, the make-up of circannual clock systems, to physiological pathways that form the basis of a certain phenotype. No matter from which discipline one approaches a problem related to phenology, it is important to keep in mind the complex relationships that underlie a phenological ‘decision’ like the laying of the first egg in spring. We have outlined an integrated framework to study phenology and we believe that this framework could function well to guide studies on a range of specific species and systems. It is not so much the lack of an existing framework as the subdivision of science that makes it hard to set up collaboration between scientists from different disciplines. Initiatives, such as the ESF/NSF/NSERC E-Bird network (Wingfield et al. 2008), can play an important role in bringing researchers together, to get acquainted with each other's terminology and state-of-the-art. In our view, the benefits of close collaboration, in particular in combined studies (see §4d), will outweigh the costs of setting up such studies.
Acknowledgements
We thank four reviewers for their valuable comments on an earlier version of this manuscript. M.E.V. was supported by a NWO-VICI grant; S.P.C. by a Léon Speeckaert Fund postdoctoral fellowship from the King Baudouin Foundation and the Belgian American Educational Foundation (BAEF); K.v.O. by a NGI-HORIZON grant.
Footnotes
One contribution of 11 to a Theme Issue ‘The role of phenology in ecology and evolution'.
References
- Baker J. R.1938The evolution of breeding seasons. In Evolution: essays on aspects of evolutionary biology (ed. Beer G. R.), pp. 161–177 London, UK: Oxford University Press [Google Scholar]
- Ball G. F., Balthazart J.2002Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In Hormones, brain and behavior (eds Pfaff D. W., Arnold A. P., Etgen A. M., Fahrbach S. E., Rubin R. T.), pp. 649–798 San Diego, CA: Academic Press [Google Scholar]
- Ball G. F., Balthazart J.2008Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Phil. Trans. R. Soc. B 363, 1699–1710 (doi:10.1098/rstb.2007.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G. F., Ketterson E. D.2008Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Phil. Trans. R. Soc. B 363, 231–246 (doi:10.1098/rstb.2007.2137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Gienapp P., Madsen J.2008The relevance of environmental conditions for departure decision changes en route in migrating geese. Ecology 89, 1953–1960 (doi:10.1890/07-1101.1) [DOI] [PubMed] [Google Scholar]
- Bentley G. E., Ball G. F.2000Photoperiod-dependent and -independent regulation of melatonin receptors in the forebrain of songbirds. J. Neuroendocrinol. 12, 745–752 (doi:10.1046/j.1365-2826.2000.00523.x) [DOI] [PubMed] [Google Scholar]
- Blondel J., Isenmann P.1979Insularity and demography of tits of the genus Parus (Aves). C. R. Hebd. Seances Acad. Sci. D 289, 161–164 [Google Scholar]
- Blondel J., Dias P. C., Maistre M., Perret P.1993Habitat heterogeneity and life-history variation of mediterranean blue tits (Parus caeruleus). Auk 110, 511–520 [Google Scholar]
- Blondel J., Dias P. C., Perret P., Maistre M., Lambrechts M. M.1999Selection-based biodiversity at a small spatial scale in a low-dispersing insular bird. Science 285, 1399–1402 (doi:10.1126/science.285.5432.1399) [DOI] [PubMed] [Google Scholar]
- Blondel J., Thomas D. W., Charmantier A., Perret P., Bourgault P., Lambrechts M. M.2006A thirty-year study of phenotypic and genetic variation of blue tits in Mediterranean habitat mosaics. Bioscience 56, 661–673 (doi:10.1641/0006-3568(2006)56[661:ATSOPA]2.0.CO;2) [Google Scholar]
- Both C., Bouwhuis S., Lessells C. M., Visser M. E.2006Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- Bradshaw W. E., Holzapfel C. M.2007Evolution of animal photoperiodism. Annu. Rev. Ecol. Evol. Syst. 38, 1–25 (doi:10.1146/annurev.ecolsys.37.091305.110115) [Google Scholar]
- Brem R. B., Kruglyak L.2005The landscape of genetic complexity across 5,700 gene expression traits in yeast. Proc. Natl Acad. Sci. USA 102, 1572–1577 (doi:10.1073/pnas.0408709102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisi R. M., Bentley G. E.2009Lab and field experiments: are they the same animal? Horm. Behav. 56, 1–10 (doi:10.1016/j.yhbeh.2009.02.010) [DOI] [PubMed] [Google Scholar]
- Caro S. P., Balthazart J., Thomas D. W., Lacroix A., Chastel O., Lambrechts M. M.2005aEndocrine correlates of the breeding asynchrony between two Corsican populations of blue tits (Parus caeruleus). Gen. Comp. Endocrinol. 140, 52–60 (doi:10.1016/j.ygcen.2004.09.016) [DOI] [PubMed] [Google Scholar]
- Caro S. P., Lambrechts M. M., Balthazart J.2005bEarly seasonal development of brain song control nuclei in male blue tits. Neurosci. Lett. 386, 139–144 (doi:10.1016/j.neulet.2005.03.074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro S. P., Lambrechts M., Chastel O., Sharp P., Thomas D. W., Balthazart J.2006Simultaneous pituitary-gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm. Behav. 50, 347–360 (doi:10.1016/j.yhbeh.2006.03.001) [DOI] [PubMed] [Google Scholar]
- Caro S. P., Charmantier A., Lambrechts M. M., Blondel J., Balthazart J., Williams T. D.2009Local adaptation of timing of reproduction: females are in the driver's seat. Funct. Ecol. 23, 172–179 (doi:10.1111/j.1365-2435.2008.01486.x) [Google Scholar]
- Charmantier A.2000Divergences adaptatives et structuration génétique chez la mésange bleue (Parus caeruleus) en Corse. Montpellier, France: Master's thesis, Université Montpellier II [Google Scholar]
- Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C.2008Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- Chong N. W., Bernard M., Klein D. C.2000Characterization of the chicken serotonin N-acetyltransferase gene—activation via clock gene heterodimer/e box interaction. J. Biol. Chem. 275, 32 991–32 998 (doi:10.1074/jbc.M005671200) [DOI] [PubMed] [Google Scholar]
- Crick H. Q. P., Sparks T. H.1999Climate change related to egg-laying trends. Nature 399, 423–424 (doi:10.1038/20839) [Google Scholar]
- Davie A., Minghetti M., Migaud H.2009Seasonal variations in clock-gene expression in Atlantic salmon (Salmo salar). Chronobiol. Int. 26, 379–395 (doi:10.1080/07420520902820947) [DOI] [PubMed] [Google Scholar]
- Dawson A., Sharp P. J.2007Photorefractoriness in birds—photoperiodic and non-photoperiodic control. Gen. Comp. Endocrinol. 153, 378–384 (doi:10.1016/j.ygcen.2007.01.043) [DOI] [PubMed] [Google Scholar]
- Dawson A., King V. M., Bentley G. E., Ball G. F.2001Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365–380 (doi:10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- Dias P. C.1996Sources and sinks in population biology. Trends Ecol. Evol. 11, 326–330 (doi:10.1016/0169-5347(96)10037-9) [DOI] [PubMed] [Google Scholar]
- Dias P. C., Verheyen G. R., Raymond M.1996Source-sink populations in Mediterranean blue tits: evidence using single-locus minisatellite probes. J. Evol. Biol. 9, 965–978 (doi:10.1046/j.1420-9101.1996.9060965.x) [Google Scholar]
- Drent P. J., van Oers K., van Noordwijk A. J.2003Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51 (doi:10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Halawani M. E., Kang S. W., Leclerc B., Kosonsiriluk S., Chaiseha Y.2008Dopamine-melatonin neurons in the avian hypothalamus and their role as photoperiodic clocks. In 9th Int. Symp. on Avian Endocrinology, pp. 123–127 Leuven, Belgium: Academic Press Inc Elsevier Science; [DOI] [PubMed] [Google Scholar]
- Farner D. S.1985Annual rhythms. Annu. Rev. Physiol. 47, 65–82 (doi:10.1146/annurev.ph.47.030185.000433) [DOI] [PubMed] [Google Scholar]
- Farner D. S., Follett B. K., King J. R., Morton M. L.1966A quantitative examination of ovarian growth in the white-crowned sparrow. Biol. Bull. 130, 67–75 (doi:10.2307/1539953) [DOI] [PubMed] [Google Scholar]
- Fidler A. E., Gwinner E.2003Comparative analysis of Avian BMALI and CLOCK protein sequences: a search for features associated with owl nocturnal behaviour. In Symp. on Function of Marine Organisms: Mechanisms of Adaption to Diverse Environments, pp. 861–874 Tokyo, Japan: Pergamon-Elsevier Science Ltd; [DOI] [PubMed] [Google Scholar]
- Follett B. K., Foster R. G., Nichols T. J.1985Photoperiodism in birds. Ciba Found. Symp. 117, 93–105 [DOI] [PubMed] [Google Scholar]
- Foster R. G., Kreitzman L.2009Seasons of life: the biological rhythms that enable living things to thrive and survive. New Haven, CT: Yale University Press [Google Scholar]
- Gienapp P., Postma E., Visser M. E.2006Why breeding time has not responded to selection for earlier breeding in a songbird population. Evolution 60, 2381–2388 [PubMed] [Google Scholar]
- Goldman B. D., Gwinner E., Karsch F. J., Saunders D., Zucker I., Ball G.2004Circannual rhythms and photoperiodism. In Chronobiology. Biological timekeeping (eds Dunlap J. C., Loros J., DeCoursey P.), pp. 107–142 Sunderland, MA: Sinauer [Google Scholar]
- Grieco F., van Noordwijk A. J., Visser M. E.2002Evidence for the effect of learning on timing of reproduction in blue tits. Science 296, 136–138 (doi:10.1126/science.1068287) [DOI] [PubMed] [Google Scholar]
- Gwinner E.1986Circannual rhythms. Heidelberg, Germany: Springer [Google Scholar]
- Gwinner E.2003Circannual rhythms in birds. Curr. Opin. Neurobiol. 13, 770–778 (doi:10.1016/j.conb.2003.10.010) [DOI] [PubMed] [Google Scholar]
- Gwinner E., Helm B.2003Circannual and circadian contributions to the timing of avian migration. In Avian migration (eds Berthold P., Gwinner E., Sonnenschein E.), pp. 81–95 Heidelberg, Germany: Springer [Google Scholar]
- Hahn T. P., MacDougall-Shackleton S. A.2008Adaptive specialization, conditional plasticity and phylogenetic history in the reproductive cue response systems of birds. Phil. Trans. R. Soc. B 363, 267–286 (doi:10.1098/rstb.2007.2139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T. P., Wingfield J. C., Mullen R., Deviche P. J.1995Endocrine bases of spatial and temporal opportunism in arctic-breeding birds. Am. Zool. 35, 259–273 [Google Scholar]
- Hahn T. P., Boswell T., Wingfield J. C., Ball G. F.1997Temporal flexibility in avian reproduction. Patterns and mechanisms. In Current ornithology, vol. 14 (eds Nolau V., Ketterson E. D., Thompson C. F.), pp. 39–80 New York, NY: Plenum [Google Scholar]
- Hau M.2007Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144 (doi:10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- Hau M., Wikelski M., Wingfield J. C.2000Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J. Exp. Zool. 286, 494–504 (doi:10.1002/(SICI)1097-010X(20000401)286:5<494::AID-JEZ7>3.0.CO;2-3) [PubMed] [Google Scholar]
- Hazlerigg D., Loudon A.2008New insights into ancient seasonal life timers. Curr. Biol. 18, R795–R804 (doi:10.1016/j.cub.2008.07.040) [DOI] [PubMed] [Google Scholar]
- Heideman P. D., Pittman J. T.2009Evolution of neuroendocrine mechanisms that regulate reproduction in white-footed mice (Peromyscus leucopus). Integr. Comp. Biol. 49, 550–562 (doi:10.1093/icb/icp014) [DOI] [PubMed] [Google Scholar]
- Heideman P. D., Bruno T. A., Singley J. W., Smedley J. V.1999Genetic variation in photoperiodism in Peromyscus leucopus: geographic variation in an alternative life-history strategy. J. Mammal. 80, 1232–1242 (doi:10.2307/1383173) [Google Scholar]
- Helfer G., Fidler A. E., Vallone D., Foulkes N. S., Brandstaetter R.2006Molecular analysis of clock gene expression in the avian brain. In 10th Congress of the European-Pineal-and-Biological-Rhythms-Society, pp. 113–127 Frankfurt, Germany: Taylor & Francis Inc; [DOI] [PubMed] [Google Scholar]
- Helm B.2009Geographically distinct reproductive schedules in a changing world: costly implications in captive stonechats. Integr. Comp. Biol. 49, 563–579 (doi:10.1093/icb/icp037) [DOI] [PubMed] [Google Scholar]
- Helm B., Gwinner E., Trost L.2005Flexible seasonal timing and migratory behavior results from stonechat breeding programs. In Workshop on analysis of hormones in dropping of birds and optimality in bird migration, vol. 1046 (eds Bauchinger U., Goymann W., JenniEiermann S.), pp. 216–227 Seewiesen, Germany: New York Acad Sciences; [DOI] [PubMed] [Google Scholar]
- Helm B., Piersma T., Van der Jeugd H.2006Sociable schedules: interplay between avian seasonal and social behaviour. Anim. Behav. 72, 245–262 (doi:10.1016/j.anbehav.2005.12.007) [Google Scholar]
- Helm B., Schwabl I., Gwinner E.2009Circannual basis of geographically distinct bird schedules. J. Exp. Biol. 212, 1259–1269 (doi:10.1242/jeb.025411) [DOI] [PubMed] [Google Scholar]
- Johnsen A., et al. 2007Avian Clock gene polymorphism: evidence for a latitudinal cline in allele frequencies. Mol. Ecol. 16, 4867–4880 (doi:10.1111/j.1365-294X.2007.03552.x) [DOI] [PubMed] [Google Scholar]
- Ketterson E. D., Nolan V.1999Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25 [DOI] [PubMed] [Google Scholar]
- Kondo N., Sekijima T., Kondo J., Takamatsu N., Tohya K., Ohtsu T.2006Circannual control of hibernation by HP complex in the brain. Cell 125, 161–172 (doi:10.1016/j.cell.2006.03.017) [DOI] [PubMed] [Google Scholar]
- Kumar V., Singh B. P., Rani S.2004The bird clock: a complex, multi-oscillatory and highly diversified system. Biol. Rhythms Res. 35, 121–144 (doi:10.1080/09291010412331313287) [Google Scholar]
- Lambrechts M. M., Dias P. C.1993Differences in the onset of laying between island and mainland Mediterranean blue tits Parus caeruleus: phenotypic plasticity or genetic differences? Ibis 135, 451–455 (doi:10.1111/j.1474-919X.1993.tb02118.x) [Google Scholar]
- Lambrechts M. M., Perret P.2000A long photoperiod overrides non-photoperiodic factors in blue tits' timing of reproduction. Proc. R. Soc. Lond. B 267, 585–588 (doi:10.1098/rspb.2000.1041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts M. M., Perret P., Blondel J.1996Adaptive differences in the timing of egg laying between different populations of birds result from variation in photoresponsiveness. Proc. R. Soc. Lond. B 263, 19–22 (doi:10.1098/rspb.1996.0004) [Google Scholar]
- Lambrechts M. M., Blondel J., Hurtrez-Boussès S., Maistre M., Perret P.1997aAdaptive inter-population differences in blue tit life-history traits on Corsica. Evol. Ecol. 11, 599–612 (doi:10.1007/s10682-997-1515-0) [Google Scholar]
- Lambrechts M. M., Blondel J., Maistre M., Perret P.1997bA single response mechanism is responsible for evolutionary adaptive variation in a bird's laying date. Proc. Natl Acad. Sci. USA 94, 5153–5155 (doi:10.1073/pnas.94.10.5153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts M. M., et al. 2004Habitat quality as a predictor of spatial variation in blue tit reproductive performance: a multi-plot analysis in a heterogeneous landscape. Oecologia 141, 555–561 (doi:10.1007/s00442-004-1681-5) [DOI] [PubMed] [Google Scholar]
- Leder E. H., Danzmann R. G., Ferguson M. M.2006The candidate gene, Clock, localizes to a strong spawning time quantitative trait locus region in rainbow trout. J. Hered. 97, 74–80 (doi:10.1093/jhered/esj004) [DOI] [PubMed] [Google Scholar]
- Le Mignon G., et al. 2009Using transcriptome profiling to characterize QTL regions on chicken chromosome 5. BMC Genomics 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells C. M.2008Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity? Phil. Trans. R. Soc. B 363, 1589–1598 (doi:10.1098/rstb.2007.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Orcutt F. S.1971Social behavior and avian sexual cycles. Scientia 106, 447–472 [Google Scholar]
- Liedvogel M., Szulkin M., Knowles S. C. L., Wood M. J., Sheldon B. C.2009Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Mol. Ecol. 18, 2444–2456 (doi:10.1111/j.1365-294X.2009.04204.x) [DOI] [PubMed] [Google Scholar]
- Lincoln G. A., Clarke I. J., Hut R. A., Hazlerigg D. G.2006Characterizing a mammalian circannual pacemaker. Science 314, 1941–1944 (doi:10.1126/science.1132009) [DOI] [PubMed] [Google Scholar]
- Lucas J. R., Freeberg T. M., Krishnan A., Long G. R.2002A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 981–992 [DOI] [PubMed] [Google Scholar]
- Lucas J. R., Freeberg T. M., Long G. R., Krishnan A.2007Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J. Comp. Physiol. A 193, 201–215 (doi:10.1007/s00359-006-0180-z) [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton S. A., Stevenson T. J., Watts H. E., Pereyra M. E., Hahn T. P.2009The evolution of photoperiod response systems and seasonal GnRH plasticity in birds. Integr. Comp. Biol. 49, 580–589 (doi:10.1093/icb/icp048) [DOI] [PubMed] [Google Scholar]
- Maney D. L., Hahn T. P., Schoech S. J., Sharp P. J., Morton M. L., Wingfield J. C.1999Effects of ambient temperature on photo-induced prolactin secretion in three subspecies of white-crowned sparrow, Zonotrichia leucophrys. Gen. Comp. Endocrinol. 113, 445–456 (doi:10.1006/gcen.1998.7219) [DOI] [PubMed] [Google Scholar]
- Martin S. L., Epperson L. E., Rose J. C., Kurtz C. C., Ane C., Carey H. V.2008Proteomic analysis of the winter-protected phenotype of hibernating ground squirrel intestine. Am. J. Physiol. Reg. I 295, R316–R328 [DOI] [PubMed] [Google Scholar]
- McGlothlin J. W., Ketterson E. D.2007Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle S. L., Follett B. K.1997Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J. Neurosci. 17, 8909–8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer T., Nienaber U., Langer U., Trillmich F.1999Temperature and timing of egg-laying of European starlings. Condor 101, 124–132 (doi:10.2307/1370453) [Google Scholar]
- Memmott J., Craze P. G., Waser N. M., Price M. V.2007Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717 (doi:10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- Menaker M.2006Circadian organization in the real world. Proc. Natl Acad. Sci. USA 103, 3015–3016 (doi:10.1073/pnas.0600360103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel A., Fabian P.1999Growing season extended in Europe. Nature 397, 659 (doi:10.1038/17709) [Google Scholar]
- Miller-Rushing A. J., Høye T. T., Inouye D. W., Post E.2010The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B 365, 3177–3186 (doi:10.1098/rstb.2010.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Nisimura T., Numata H.2005A phase response curve for circannual rhythm in the varied carpet beetle Anthrenus verbasci. J. Comp. Physiol. A 191, 883–887 (doi:10.1007/s00359-005-0012-6) [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Nisimura T., Numata H.2009Circannual pupation rhythm in the varied carpet beetle Anthrenus verbasci under different nutrient conditions. Entomol. Sci. 12, 370–375 (doi:10.1111/j.1479-8298.2009.00349.x) [Google Scholar]
- Moore M. C.1983Effect of female sexual displays on the endocrine physiology and behavior of male white-crowned sparrows, Zonotrichia leucophrys. J. Zool. 199, 137–148 (doi:10.1111/j.1469-7998.1983.tb02085.x) [Google Scholar]
- Moore I. T., Bonier F., Wingfield J. C.2005Reproductive asynchrony and population divergence between two tropical bird populations. Behav. Ecol. 16, 755–762 (doi:10.1093/beheco/ari049) [Google Scholar]
- Moore I. T., Bentley G. E., Wotus C., Wingfield J. C.2006Photoperiod-independent changes in immunoreactive brain gonadotropin-releasing hormone (GnRH) in a free-living, tropical bird. Brain Behav. Evol. 68, 37–44 (doi:10.1159/000093059) [DOI] [PubMed] [Google Scholar]
- Mrosovsky N.1970Mechanism of hibernation cycles in ground squirrels: circannian rhythm or sequence of stages. Penn. Acad. Sci. 44, 172–175 [Google Scholar]
- Mrosovsky N.1986Thermal effects on the periodicity, phasing, and persistence of circannual cycles. In Living in the cold: physiological biochemical adaptations (eds Heller H. C., Musacchia X. J., Wang L. C. H.), pp. 403–410 New York, NY: Elsevier [Google Scholar]
- Nisimura T., Numata H.2001Endogenous timing mechanism controlling the circannual pupation rhythm of the varied carpet beetle Anthrenus verbasci. J. Comp. Physiol. A 187, 433–440 (doi:10.1007/s003590100215) [DOI] [PubMed] [Google Scholar]
- Notter D. R.2008Genetic aspects of reproduction in sheep. In 16th Int. Congress on Animal Reproduction, pp. 122–128 Budapest, Hungary: Blackwell Publishing; [DOI] [PubMed] [Google Scholar]
- Notter D. R., Cockett N. E.2003Opportunities for detection and use of QTL influencing seasonal reproduction in sheep: a review. In 3rd Int. Workshop on Major Genes and QTL in Sheep and Goats, pp. S39–S53 Toulouse, France: E D P Sciences; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey D. H., Postma E., Gienapp P., Visser M. E.2005Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- O'Brien S., Hau M.2005Food cues and gonadal development in neotropical spotted antbirds (Hylophylax naevioides). J. Ornithol. 146, 332–337 [Google Scholar]
- Ono H., Nakao N., Yoshimura T.2009Identification of the photoperiodic signaling pathway regulating seasonal reproduction using the functional genomics approach. Gen. Comp. Endocrinol. 163, 2–6 (doi:10.1016/j.ygcen.2008.11.017) [DOI] [PubMed] [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Partecke J., Vańt Hof T. J., Gwinner E.2004Differences in the timing of reproduction between urban and forest European blackbirds (Turdus merula)—result of phenotypic flexibility or genetic differences? Proc. R. Soc. Lond. B 271, 1995–2001 (doi:10.1098/rspb.2004.2821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partecke J., Vańt Hof T. J., Gwinner E.2005Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 36, 295–305 (doi:10.1111/j.0908-8857.2005.03344.x) [Google Scholar]
- Paul M., Zucker I., Schwartz W. J.2008Tracking the seasons: the internal calendars of vertebrates. Phil. Trans. R. Soc. B 363, 341–361 (doi:10.1098/rstb.2007.2143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfito N., Bentley G. E.2009Opportunism, photoperiodism, and puberty: different mechanisms or variations on a theme? Integr. Comp. Biol. 49, 538–549 (doi:10.1093/icb/icp052) [DOI] [PubMed] [Google Scholar]
- Perfito N., Meddle S. L., Tramontin A. D., Sharp P. J., Wingfield J. C.2005Seasonal gonadal recrudescence in song sparrows: response to temperature cues. Gen. Comp. Endocrinol. 143, 121–128 (doi:10.1016/j.ygcen.2005.03.004) [DOI] [PubMed] [Google Scholar]
- Perfito N., Bentley G., Hau M.2006Tonic activation of brain GnRH immunoreactivity despite reduction of peripheral reproductive parameters in opportunistically breeding zebra finches. Brain Behav. Evol. 67, 123–134 (doi:10.1159/000090977) [DOI] [PubMed] [Google Scholar]
- Perfito N., Kwong J. M. Y., Bentley G. E., Hau M.2008Cue hierarchies and testicular development: is food a more potent stimulus than day length in an opportunistic breeder (Taeniopygia g. guttata)? Horm. Behav. 53, 567–572 (doi:10.1016/j.yhbeh.2008.01.002) [DOI] [PubMed] [Google Scholar]
- Pigliucci M.2005Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486 (doi:10.1016/j.tree.2005.06.001) [DOI] [PubMed] [Google Scholar]
- Post E., Forchhammer M. C.2008Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375 (doi:10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido F.2007Phenotypic changes in spring arrival: evolution, phenotypic plasticity, effects of weather and condition. Clim. Res. 35, 5–23 (doi:10.3354/cr00711) [Google Scholar]
- Ryan M. J., Perrill S. A., Wilczynski W.1992Auditory tuning and call frequency predict population-based mating preferences in the cricket frog Acris crepitans. Am. Nat. 139, 1370–1383 (doi:10.1086/285391) [Google Scholar]
- Salvante K. G., Walzem R. L., Williams T. D.2007What comes first, the zebra finch or the egg: temperature-dependent reproductive, physiological and behavioural plasticity in egg-laying zebra finches. J. Exp. Biol. 210, 1325–1334 (doi:10.1242/jeb.02745) [DOI] [PubMed] [Google Scholar]
- Schwartz M. D.(ed.)2003Phenology: an integrative environmental science. Norwell, MA: Kluwer Academic Publishers [Google Scholar]
- Sharp P. J.2005Photoperiodic regulation of seasonal breeding in birds. Trends Comp. Endocrinol. Neurobiol. 1040, 189–199 [DOI] [PubMed] [Google Scholar]
- Silverin B., Viebke P. A.1994Low-temperatures affect the photoperiodically induced LH and testicular cycles differently in closely-related species of tits (Parus spp). Horm. Behav. 28, 199–206 (doi:10.1006/hbeh.1994.1017) [DOI] [PubMed] [Google Scholar]
- Silverin B., Wingfield J., Stokkan K. A., Massa R., Jarvinen A., Andersson N. A., Lambrechts M., Sorace A., Blomqvist D.2008Ambient temperature effects on photo-induced gonadal cycles and lehormonal secretion patterns in great tits from three different breeding latitudes. Horm. Behav. 54, 60–68 (doi:10.1016/j.yhbeh.2008.01.015) [DOI] [PubMed] [Google Scholar]
- Singer M. C., Parmesan C.2010Phenological asynchrony between herbivorous insects and their hosts: signal of climate change or pre-existing adaptive strategy? Phil. Trans. R. Soc. B 365, 3161–3176 (doi:10.1098/rstb.2010.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small T. W., Sharp P. J., Deviche P.2007Environmental regulation of the reproductive system in a flexibly breeding Sonoran Desert bird, the rufous-winged sparrow, Aimophila carpalis. Horm. Behav. 51, 483–495 (doi:10.1016/j.yhbeh.2007.01.004) [DOI] [PubMed] [Google Scholar]
- Sockman K. W., Williams T. D., Dawson A., Ball G. F.2004Prior experience with photostimulation enhances photo-induced reproductive development in female European starlings: a possible basis for the age-related increase in avian reproductive performance. Biol. Reprod. 71, 979–986 (doi:10.1095/biolreprod.104.029751) [DOI] [PubMed] [Google Scholar]
- Stevenson T. J., Bentley G. E., Ubuka T., Arckens L., Hampson E., MacDougall-Shackleton S. A.2008Effects of social cues on GnRH-I, GnRH-II, and reproductive physiology in female house sparrows (Passer domesticus). Gen. Comp. Endocrinol. 156, 385–394 (doi:10.1016/j.ygcen.2008.01.015) [DOI] [PubMed] [Google Scholar]
- Tauber E., Kyriacou C. P.2005Molecular evolution and population genetics of circadian clock genes. In Circadian Rhythms 393, pp. 797–817 San Diego, CA: Elsevier Academic Press Inc; [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Blondel J., Perret P., Lambrechts M. M., Speakman J. R.2001Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600 (doi:10.1126/science.1057487) [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Ubuka T., Yin H., Osugi T., Ukena K., Bentley G. E., Sharp P. J., Wingfield J. C.2007Discovery of gonadotropin-inhibitory hormone in a domesticated bird, its mode of action and functional significance. J. Ornithol. 148, S515–S520 [DOI] [PubMed] [Google Scholar]
- Tsutsui K., et al. 2009A new key neurohormone controlling reproduction, gonadotrophin-inhibitory hormone in birds: discovery, progress and prospects. J. Neuroendocrinol. 21, 271–275 (doi:10.1111/j.1365-2826.2009.01829.x) [DOI] [PubMed] [Google Scholar]
- Ubuka T., McGuire N. L., Calisi R. M., Perfito N., Bentley G. E.2008The control of reproductive physiology and behavior by gonadotropin-inhibitory hormone. Integr. Comp. Biol. 48, 560–569 (doi:10.1093/icb/icn019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asch M., Tienderen P. H., Holleman L. J. M., Visser M. E.2007Predicting adaptation of phenology in response to climate change, an insect herbivore example. Global Change Biol. 13, 1596–1604 (doi:10.1111/j.1365-2486.2007.01400.x) [Google Scholar]
- Visser M. E.2008Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Both C.2005Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Lambrechts M. M.1999Information constraints in the timing of reproduction in temperate zone birds: great and blue tits. In Proc. 22 Int. Ornithol. Congr. Durban (eds Adams N. J., Slotow R. H.), pp. 249–264 Johannesburg, South Africa: BirdLife South Africa [Google Scholar]
- Visser M. E., van Noordwijk A. J., Tinbergen J. M., Lessells C. M.1998Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 (doi:10.1098/rspb.1998.0514) [Google Scholar]
- Visser M. E., Both C., Lambrechts M. M.2004Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 35, 89–110 (doi:10.1016/S0065-2504(04)35005-1) [Google Scholar]
- Visser M. E., Holleman L. J. M., Gienapp P.2006Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 (doi:10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- Visser M. E., Holleman L. J. M., Caro S. P.2009Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331 (doi:10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleck C. M., Priedkalns J.1985Reproduction in zebra finches—hormone levels and effect of dehydration. Condor 87, 37–46 (doi:10.2307/1367129) [Google Scholar]
- Voigt C., Goymann W., Leitner S.2007Green matters! Growing vegetation stimulates breeding under short-day conditions in wild canaries (Serinus canaria). J. Biol. Rhythms 22, 554–557 (doi:10.1177/0748730407306928) [DOI] [PubMed] [Google Scholar]
- Wada M.1993Low-temperature and short days together induce thyroid activation and suppression of LH-release in Japanese quail. Gen. Comp. Endocrinol. 90, 355–363 (doi:10.1006/gcen.1993.1091) [DOI] [PubMed] [Google Scholar]
- Wada M., Hatanaka F., Tsuyoshi H., Sonoda Y.1990Temperature modulation of photoperiodically induced LH-secretion and its termination in Japanese quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 80, 465–472 (doi:10.1016/0016-6480(90)90195-R) [DOI] [PubMed] [Google Scholar]
- Wikelski M., Martin L. B., Scheuerlein A., Robinsoni M. T., Robinson N. D., Heim B., Hau M., Gwinner E.2008Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Phil. Trans. R. Soc. B 363, 411–423 (doi:10.1098/rstb.2007.2147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczek A. M., Burghardt L. T., Cobb A. R., Cooper M. D., Welch S. M., Schmitt J.2010Genetic and physiological bases for phenological responses to current and predicted climates. Phil. Trans. R. Soc. B 365, 3129–3147 (doi:10.1098/rstb.2010.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. D.2008Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean'. Phil. Trans. R. Soc. B 363, 1687–1698 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. C.2008Comparative endocrinology, environment and global change. Gen. Comp. Endocrinol. 157, 207–216 (doi:10.1016/j.ygcen.2008.04.017) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Jacobs J. D.1999The interplay of innate and experiential factors regulating the life history cycle of birds. In Proc. 22nd Int. Ornithol. Congr. Durban (eds Adams N. J., Slotow R. H.), pp. 2417–2443 Johannesburg, South Africa: BirdLife South Africa [Google Scholar]
- Wingfield J. C., Kenagy G. J.1991Natural regulation of reproductive cycles. In Vertebrate endocrinology: fundamentals and biomedical implications, vol. 4 (eds Pawg P. K. T., Schreibman M. P.), pp. 181–241 New York, NY: Academic Press Inc [Google Scholar]
- Wingfield J. C., Moore M. C.1987Hormonal, social, and environmental factors in the reproductive biology of free-living male birds. In Psychology of reproduction: an evolutionary perspective (ed. Crews D.), pp. 148–175 Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- Wingfield J. C., Hahn T. P., Levin R., Honey P.1992Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 261, 214–231 (doi:10.1002/jez.1402610212) [Google Scholar]
- Wingfield J. C., Hahn T. P., Wada M., Astheimer L. B., Schoech S.1996Interrelationship of day length and temperature on the control of gonadal development, body mass, and fat score in white-crowned sparrows, Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 101, 242–255 (doi:10.1006/gcen.1996.0027) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Hahn T. P., Wada M., Schoech S. J.1997Effects of day length and temperature on gonadal development, body mass, and fat depots in white-crowned sparrows, Zonotrichia leucophrys pugetensis. Gen. Comp. Endocrinol. 107, 44–62 (doi:10.1006/gcen.1997.6894) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Breuner C. W., Jacobs J. D., Lynn S., Maney D. L., Ramenofsky M., Richardson R. D.1998Ecological bases of hormone-behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- Wingfield J. C., Hahn T. P., Maney D. L., Schoech S. J., Wada M., Morton M. L.2003Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows, Zonotrichia leucophrys oriantha. Gen. Comp. Endocrinol. 131, 143–158 (doi:10.1016/S0016-6480(02)00648-2) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Visser M. E., Williams T. D.2008Introduction. Integration of ecology and endocrinology in avian reproduction: a new synthesis. Phil. Trans. R. Soc. B 363, 1581–1588 (doi:10.1098/rstb.2007.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo S., Watanabe M., Okabayashi N., Ebihara S., Yoshimura T.2003Circadian clock genes and photoperiodism: comprehensive analysis of clock genes expression in the mediobasal hypothalamus, the suprachiasmatic nucleus and the pineal gland of Japanese quail under various light schedules. Endocrinology 144, 3742–3748 (doi:10.1210/en.2003-0435) [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Suzuki Y., Makino E., Suzuki T., Kuroiwa A., Matsuda Y., Namikawa T., Ebihara S.2000Molecular analysis of avian circadian clock genes. Mol. Brain Res. 78, 207–215 (doi:10.1016/S0169-328X(00)00091-7) [DOI] [PubMed] [Google Scholar]
- Zandt H., Strijkstra A., Blondel J., van Balen J. H.1990Food in two mediterranean blue tit populations: do differences in caterpillar availability explain differences in the timing of the breeding season. In Population biology of Passerine birds. An integrated approach (eds Blondel J., Gosler A., Lebreton J. D., McCleery R.), pp. 145–155 Berlin, Germany: Springer [Google Scholar]
- Zann R. A., Morton S. R., Jones K. R., Burley N. T.1995The timing of breeding by zebra finches in relation to rainfall in central Australia. Emu 95, 208–222 [Google Scholar]
- Zou W., Zeng Z. B.2009Multiple interval mapping for gene expression QTL analysis. Genetica 137, 125–134 (doi:10.1007/s10709-009-9365-z) [DOI] [PubMed] [Google Scholar]