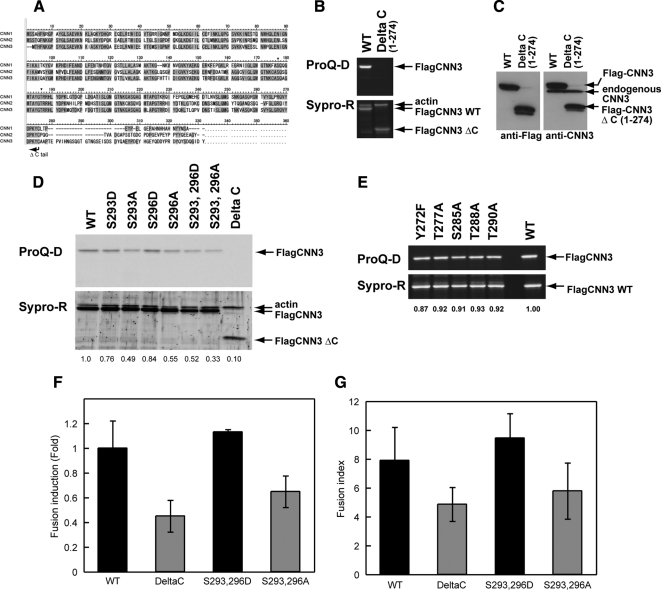
Figure 4.
CNN3 phosphorylation in BeWo cell fusion. (A) Comparison of the amino acid sequences of human CNN family proteins. CNN1 phosphorylation sites are indicated by asterisks. The sequence of ΔC mutant is truncated after Cys274 (arrow). (B) HEK293 cells transiently expressing WT or ΔC CNN3. Flag-CNN3 with a wild or Δ C sequence was recovered by immunoprecipitation from cell lysate using anti-Flag-agarose beads. The eluate was subjected to SDS-PAGE, and the phosphorylated CNN3s were visualized by ProQ Diamond phospho-specific staining (upper panel) followed by Sypro Ruby protein staining (lower panel). Note that the ΔC mutant was not phosphorylated but retained the actin-binding property. (C) Flag-tagged WT or ΔC CNN3 identified by Western blotting using anti-FLAG (left panel) or anti-CNN3 (right panel) antibodies. Each 20-μg sample of HEK293 cell lysate was loaded. (D and E) Phosphorylation levels of WT or mutant Flag-CNN3 transiently expressed in HEK293 cells. Flag-CNN3 was immunopurified using anti–Flag-agarose beads and was subjected to SDS-PAGE followed by ProQ Diamond phospho-specific staining. The ratio of phosphorylated and total CNN3 was calculated and indicated below each lane, showing decreased phosphorylation of S293A and S296A CNN3s. (F and G) BeWo cell fusion at 96 h after forskolin treatment in various mutants. The fusion frequency was counted as “fusion induction” (F), and the degree of maturation was calculated as “fusion index” (G). Data from three independent experiments were averaged, and standard deviations are indicated by error bars.