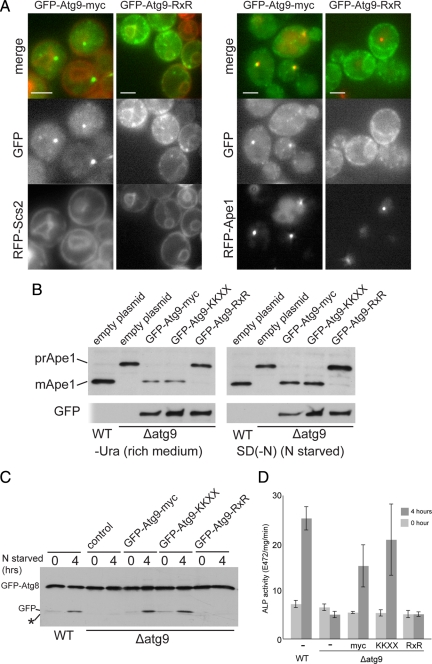
Figure 1.
Atg9 cannot get directly from the ER to the forming autophagosome. (A) Fluorescence micrographs of yeast expressing GFP-Atg9 with a C-terminal myc-tag followed by nothing or an RXR motif [KLRRRRI (Michelsen et al., 2007)]. The fusions were expressed with a TPI1 promoter from a centromeric vector and the cells also express either RFP-Ape1, or the ER protein RFP-Scs2. The RXR motif increases the amount of Atg9 in the ER and reduces that present at aggregates of mRFP-Ape1. Scale bars = 2 μm. (B) Anti-Ape1 immunoblots of lysates from wild-type cells, or from an ATG9 deletion strain transformed with either an empty plasmid, or the same GFP-Atg9 plasmids shown in A, and also one with the C-terminal myc-tag followed by a KKXX motif (SKKSL). Cells were harvested after growth in rich medium or after four hours of nitrogen starvation to induce autophagy. Attachment of the RXR motif to Atg9 blocks its activity under both conditions. (C) Anti-GFP immunoblots of lysates from cells expressing GFP-Atg8 from the ATG8 promoter on a CEN plasmid. The yeast strains are as in A and B but with the ATG9 fusions integrated into the genome. The cells were grown to midlog phase and either harvested (0) or starved for nitrogen for 4 h. Deletion of Atg9 prevents delivery of GFP-Atg8 to the vacuole and release of free GFP. (D) Alkaline phosphatase activity in strains expressing a cytosolic form of Pho8 (Pho8Δ60) that lacks a transmembrane domain and so only becomes active upon delivery to the vacuole by bulk autophagy (Noda and Ohsumi, 1998). Strains are as in C but with PHO13 deleted and PHO8 truncated to PHO8Δ60 by integration of TDH3 promoter. Cells were grown and starved for 4 h as in C (error bars indicate SD of three independent experiments).