Abstract
Astronomical observations have shown that carbonaceous compounds in the gas and solid state, refractory and icy are ubiquitous in our and distant galaxies. Interstellar molecular clouds and circumstellar envelopes are factories of complex molecular synthesis. A surprisingly large number of molecules that are used in contemporary biochemistry on Earth are found in the interstellar medium, planetary atmospheres and surfaces, comets, asteroids and meteorites, and interplanetary dust particles. In this article we review the current knowledge of abundant organic material in different space environments and investigate the connection between presolar and solar system material, based on observations of interstellar dust and gas, cometary volatiles, simulation experiments, and the analysis of extraterrestrial matter. Current challenges in astrochemistry are discussed and future research directions are proposed.
Complex organic molecules are present in cosmic dust, comets, and meteorites, which could have delivered material necessary for the emergence of life on Earth and other planets.
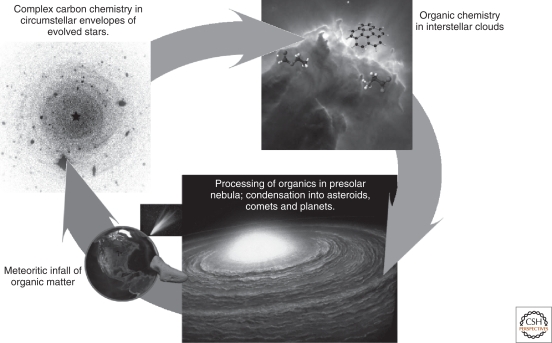
Carbon is a key element in the evolution of prebiotic material (Henning and Salama 1998), and becomes biologically interesting in compounds with nitrogen, oxygen and hydrogen. Our understanding of the evolution of organic molecules—including such compounds—and their voyage from molecular clouds to the early solar system and Earth provides important constraints on the emergence of life on Earth and possibly elsewhere (Ehrenfreund and Charnley 2000). Figure 1 shows the cycle of organic molecules in the universe. Gas and solid-state chemical reactions form a variety of organic molecules in circumstellar and interstellar environments. During the formation of the solar system, this interstellar organic material was chemically processed and later integrated in the presolar nebula from which planets and small solar system bodies formed. The remnant planetesimals in the form of comets and asteroids impacted the young planets in the early history of the solar system (Gomes et al. 2005). The large quantities of extraterrestrial material delivered to young planetary surfaces during the heavy bombardment phase may have played a key role in life's origin (Chyba and Sagan 1992, Ehrenfreund et al. 2002). How elements are formed, how complex carbonaceous molecules in space are, what their abundance is and on what timescales they form are crucial questions within cosmochemistry.
Figure 1.
Carbon pathways between interstellar and circumstellar regions and the forming solar system.
Inventory of Cosmic Carbon
Carbon is found in space in all its allotropic forms: diamond, graphite, and fullerene (Cataldo et al. 2004). Astronomical observations in the last decade have shown that carbonaceous compounds (gaseous molecules and solids) are ubiquitous in our own as well as in distant galaxies (Ehrenfreund et al. 2006a). The first chemical enrichment of the universe may likely be connected to the first generation of stars (Spaans 2004). Large carbon abundances are already extrapolated from observations of the strong C[II] and CO lines in the hosts of the most distant quasars (Bertoldi et al. 2003).
Carbon in space was first produced in stellar interiors in fusion reactions and was later ejected into interstellar and intergalactic space during stellar collapse and supernova explosions. In the denser regions of interstellar space, the so-called interstellar clouds, active chemical pathways form simple and complex carbon molecules from carbon atoms (van Dishoeck and Blake 1998). Circumstellar envelopes are regarded as the largest factories of carbon chemistry in space (Kwok 2004, 2009).
Organic molecules in the solar system are found in planetary atmospheres and on the surface of many outer solar system moons (e.g., Cruikshank et al. 2005; Raulin 2008; Lorenz et al. 2008). More than 50 molecules have been identified in cometary comae (Crovisier et al. 2009). Many small organic molecules observed in cometary comae probably originate wholly or partially from the decomposition of larger molecules or particles, indicating that large polymers such as polyoxymethylene and HCN-polymers are present in comets (Ehrenfreund et al. 2004; Cottin and Fray 2008). Carbonaceous chondrites (meteorites) and micrometeorites do contain a variety of organics (e.g., see Alexander et al. 2007; Sephton 2002; Septhon and Botta 2005 for reviews). They are fragments of cometary and asteroidal bodies. Investigating their organic composition often indicates the nature of the parental body (Hiroi et al. 1993; Ehrenfreund et al. 2001; Nesvorny et al. 2009).
Cosmic Cycling of Organics
The interstellar medium, the space between the stars, is composed primarily of H and He and constitutes a few percent of the galactic mass. Interstellar material is dominated by interstellar gas (99%). The remaining 1% is composed of solid silicate and carbon-based µm-sized dust particles present throughout interstellar clouds that provide surfaces for accretion of gas phase species and subsequent grain surface chemistry (Ehrenfreund and Charnley 2000; Ehrenfreund and Fraser 2003). Fundamental physical parameters such as temperature and density vary strongly across the spectrum of interstellar regions. The diversity of interstellar clouds results from energy injected by supernova shockwaves and stellar outflows and radiative losses (Wolfire et al. 2003). The most recent classification by Wooden et al. (2004) describes in detail very low-density hot gas, environments with warm intercloud gas, and regions with denser and colder material, see Table 1. Cosmic abundances in the interstellar medium are derived by measuring elemental abundances in stellar atmospheres. These cosmic elemental abundances determine the amount of elements available for the formation of molecules and particles. Gas phase and solid-state reactions and gas-grain interactions lead to the formation of complex molecules. H2 is by far the most abundant molecule in cold interstellar regions, followed by CO, the most abundant carbon-containing species, with CO/H2 ∼10−4.
Table 1.
Phases of the interstellar medium (adapted from Wooden et al. 2004).
| ISM component | Designation | Temperature (K) | Density [cm−3] |
|---|---|---|---|
| Hot ionized medium | coronal gas | 106 | 0.003 |
| Warm ionized medium | diffuse ionized gas | 104 | >10 |
| Warm neutral medium | intercloud HI | 104 | 0.1 |
| Atomic cold neutral medium | diffuse clouds | 100 | 10–100 |
| Molecular cold neutral medium | dark clouds, molecular clouds, dense clouds | <50 | 103–105 |
| Molecular hot cores | protostellar cores | 100–300 | >106 |
Adapted from Wooden et al. 2004.
In cold dark clouds with a temperature of 3–10 K the sticking coefficient of most atoms and molecules is close to unity and all species (except H2 and He) freeze out (Ehrenfreund and Charnley 2000). At 10 K only H, D, C, O, and N atoms have sufficient mobility to interact on the surfaces of grains. Dark clouds offer a protected environment for the formation of larger molecules. Those regions have a rather high density (∼106/ cm3) and experience a low radiation field of ∼103 photons cm2/s induced by cosmic rays (Prasad and Tarafdar 1983).
The diffuse interstellar medium is characterized by a low density (∼103 atoms/cm3) and temperatures ≤100 K. Diffuse clouds are filamentary structures that surround cold dense interstellar regions. Ices are not present in those regions and a strong radiation field of ∼108 photons/cm2/s (Mathis et al. 1983) dominates the formation and evolution of molecules and larger structures. Small carbonaceous molecules in the gas phase are easily destroyed by radiation. Atoms with ionization potentials less than 13.6 eV are photoionized. The identification of many small molecules in dense clouds implies that their destruction is well balanced by active formation routes. Ion-molecule reactions, dissociative recombination with electrons, radiative association reactions and neutral-neutral reactions contribute to gas phase processes and influence interstellar chemistry (Snow and McCall 2006).
“Stardust,” in the form of dust and molecules, is injected by stellar sources in their late stage of evolution into interstellar clouds. Whereas the low temperature dust in dense interstellar clouds is covered by ice that experiences low UV radiation flux, dust in diffuse clouds is strongly processed by UV radiation and shocks. The distinct and surprising differences in the dust component of dense and diffuse interstellar clouds do not suggest a rapid cycling of cloud material (Chiar and Pendleton 2008). Previous ideas of periodical cycling of dust are currently revisited (e.g., Chiar et al. 2007). Understanding interstellar cloud evolution and dust cycling provides important insights into the nature of the material that is later incorporated into proto-planetary material (see Solar System Formation below).
The Conditions on the Young Terrestrial Planets
Planet Earth was formed through a hot accretion process that allowed only the rocky material from the inner solar system to survive. Ices were sublimed and existing carbon material (volatile and refractory) pyrolized. Therefore, organic molecules found on terrestrial planets must have formed after the planetary surface cooled, or were delivered via impacts by small bodies. All terrestrial planets have been seeded with organic compounds through the impact of small bodies during solar system formation. Part of this material may have been important starting material for life (Chyba et al. 1990; Ehrenfreund et al. 2002). At present, very little data are available regarding the atmospheric, oceanic, or geological conditions on the prebiological Earth. It is assumed, however, that conditions on the young Earth were very hostile due to volcanism, radiation, and bombardment by comets and asteroids. Primitive life, in the form of bacteria, emerged approximately 3.5 billion years ago (Derenne et al. 2008).
Earth provides an ideal environment for life to persist: Dynamic processes in the Earth's interior have established a magnetosphere that protects life from harmful cosmic ray particles impinging the Earth's atmosphere; in turn, the atmosphere shields life from radiation and allows for a stable climate and temperature cycle. A brief look at our planetary neighbors shows that Venus, with an average surface temperature of 500°C and Mars, with an average surface temperature of −60°C and a thin atmosphere, are both apparently unable to sustain life at their surfaces (Lammer et al. 2009). Oxidizing compounds are held responsible for the degradation of organics in the Martian soil (Quinn et al. 2005).
BACKGROUND
Formation of Carbon Compounds in Space Environments
Interstellar molecules can be identified through their electronic transition in the UV and optical part of the spectrum or in the infrared range through vibrational transitions. Molecules with a dipole moment display rotational lines that can be observed at radio wavelength. At the beginning of the 20th century, astronomers were skeptical about the presence of molecular species in the interstellar medium. However, the presence of simple molecules such as CN, CH, and CH+ was confirmed in the 1940s by optical absorption spectroscopy. Telescopes capable of observing rotational molecular emissions at millimeter wavelengths in the 1970s allowed the discovery of many molecules.1
Carbon chemistry occurs efficiently in diffuse interstellar clouds. Simple molecules such as CO, CH, CN, OH, C2, C3, and others can be observed throughout the electromagnetic spectrum (Liszt and Lucas 2000). Cosmic dust models indicate that the majority of carbon in diffuse clouds (up to 80%) is incorporated into carbonaceous grains and gaseous polycyclic aromatic hydrocarbon molecules (PAHs) (Pendleton and Allamandola 2002; Snow and McCall 2006).
Amorphous carbon, hydrogenated amorphous carbon, diamond, refractory organics, and carbonaceous networks such as coal, soot, graphite, quenched-carbonaceous condensates, and others have been proposed as possible solid carbon components of interstellar clouds (see Henning and Salama, 1998, Ehrenfreund and Charnley, 2000, Pendleton and Allamandola 2002, Tielens 2008). Recent spectroscopic evidence indicates that carbonaceous grains are predominantly made of amorphous carbon (Mennella et al. 1998; Henning and Mutschke 2004).
PAHs are observed widely distributed in galactic and extragalactic regions (Genzel et al. 1998; Peeters et al. 2004a,b; Smith et al. 2007; Tielens 2008). Their abundance is estimated to be 5×10−7 (with respect to H). Laboratory studies and theoretical calculations have provided important insights into their size and charge state distribution (e.g., Salama 1999; Allamandola et al. 1999; Ruiterkamp et al. 2005a; Bauschlicher et al. 2008, 2009).
Observations at infrared, radio, millimeter, and submillimeter frequencies show that a large variety of organic molecules are present in the dense interstellar gas (www.astrochemistry.net lists more than 150 molecules, Charnley et al. 2003). These include organics such as nitriles, aldehydes, alcohols, acids, ethers, ketones, amines, and amides, as well as long-chain hydrocarbons. Infrared observations of icy dust particles in the last decade with the Infrared Space Observatory, the Spitzer Space telescope and complementary ground based observations revealed a large variety of organic compounds. The nature of specific ice mixtures and the abundance of the individual compounds in grain mantles have been measured with unprecedented accuracy (Gibb et al. 2004; van Dishoeck 2004). In dense clouds, atoms accreted on dust particles can enter reaction pathways such as exothermic hydrogenation reactions, which result in the formation of the simplest mantle molecules (water, ammonia, methane etc.). The main species observed in interstellar ice mantles are H2O, CO2, CO, and CH3OH, with smaller admixtures of CH4, NH3, H2CO, and HCOOH (Gibb et al. 2004).
The circumstellar envelopes of carbon-rich stars are the heart of the most complex carbon chemistry that is analogous to soot formation in candle flames or industrial smoke stacks (Henning and Mutschke 2004). Laboratory simulations of gas-phase condensation reactions such as laser pyrolysis and laser ablation showed that the temperature in the condensation zone determines the formation pathway of carbonaceous particles. At temperatures lower than 1700 K, the condensation by-products are mainly PAHs with three to five aromatic rings (Jäger et al. 2008). At condensation temperatures higher than 3500 K, fullerene-like carbon grains and fullerene compounds are formed. Molecular synthesis may occur in the circumstellar environment on timescales as short as several hundred years (Kwok 2004, 2009).
Solar System Formation
Interstellar matter provides the raw material for the formation of stars and planets. Approximately 4.6 billion years ago, the gravitational collapse of an interstellar cloud led to the formation of a protosolar disk (the solar nebula) with a central condensation developing into our Sun. Interstellar dust clumped together to form small particles in the solar nebula that grew bigger, accreted more and more material, eventually forming planets and small bodies such as comets and asteroids (Boss 2004; Blum 2004).
Volatile and robust carbon compounds residing in interstellar clouds were recycled during solar system formation (Ciesla 2008). The dynamic environment of the solar nebula with the simultaneous presence of gas, particles, and energetic processes, including shock waves, lightning, and radiation can trigger a rich organic chemistry leading to organic molecules (Chick and Cassen 1997, Gorti et al. 2009). Turbulent motion led to radial mixing of the products within the disk (Markwick and Charnley 2004; Visser et al. 2007; Dullemond et al. 2008).
The carbonaceous inventory of our solar system therefore contains highly processed material that was exposed to high temperatures and radiation, newly formed compounds, and some relatively pristine material with significant interstellar heritage. Organic compounds observed or sampled from our solar system, such as planetary surfaces/atmospheres, comets, and interplanetary dust, thus hold clues to processes that occurred during the origin of our solar system (Ehrenfreund and Charnley 2000; Cruikshank et al. 2005; Septhon and Botta 2005; Raulin 2008).
Extraterrestrial Delivery
The large quantities of extraterrestrial material delivered to young terrestrial planetary surfaces in the early history of our solar system may have provided the material necessary for the emergence of life (Chyba et al. 1992; Ehrenfreund et al. 2002). Comets are predominantly icy bodies containing some silicates and refractory organic material formed in the region beyond Jupiter (Greenberg 1998; Di Santi and Mumma 2009). The NASA Stardust comet sample-return mission captured cometary dust intact at a velocity of 6.1 km/s at a distance of about 300 km from the nucleus of comet Wild-2. Most of the >5 µm solid particles collected by the mission are mineral grains or assemblages of high-temperature minerals that condense at 1400 K or above. The data provided evidence for radial transport of large solid grains from the center of the solar nebula to the Kuiper belt (Brownlee et al. 2006). Comets probably contributed most of the carbonaceous compounds during the heavy bombardment phase 4.5–4 billion years ago (Ehrenfreund et al. 2002). Fragments of asteroids and comets such as interplanetary dust particles (IDPs) and carbonaceous meteorites were probably among the other major extraterrestrial contributors of carbon (Chyba et al. 1990).
Carbonaceous meteorites contain a substantial amount of carbon (up to 5% by weight) most of which is in organic compounds (Mullie and Reisse 1987); inorganic carbon is present as diamond, graphite and carbonate materials. They exhibit evidence of thermal and aqueous alteration believed to have occurred on their parent bodies (Sephton 2002; Botta and Sephton 2005; Martins et al. 2007). In the soluble fraction of the Murchison meteorite, more than 70 extraterrestrial amino acids have been identified in addition to many other organic compounds, including N-heterocycles, carboxylic acids, sulfonic and phosphonic acids, and aliphatic and aromatic hydrocarbons (Cronin et al. 1993; Martins et al. 2007, 2008). However, the major carbon component in meteorite samples is composed of a macromolecular organic fraction (Alexander et al. 2007). Although several classes of organic compounds important in contemporary biochemistry are found in the interstellar medium, comets and meteorites, the dominant form of carbonaceous material that will be delivered is likely aromatic in nature.
RECENT RESULTS OF SELECTED ABUNDANT ORGANICS IN SPACE FROM OBSERVATIONS, LABORATORY STUDIES AND MODELLING
Gas Phase Molecules
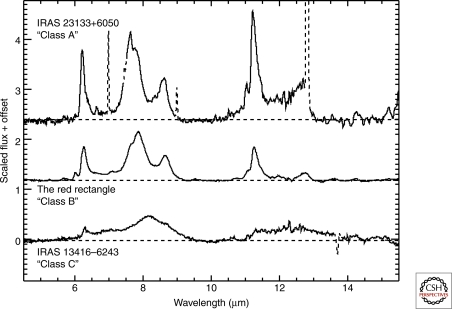
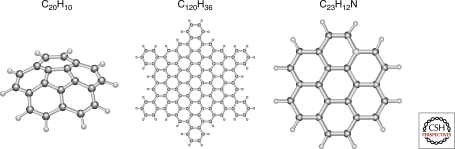
Polycyclic aromatic hydrocarbons (PAHs) are composed of aromatic rings and are characterized by a high stability against radiation, in particular when in pericondensed form. PAHs are observed widely distributed in galactic and extragalactic regions and have been identified by their characteristic emission features in the near and mid infrared at 3.3, 6.2, 7.7, 8.6, 11.2, and 12.7 µm, representative of vibrational modes of C–C and C–H bonds, see Figure 2. They are the most abundant organic molecules in the gas phase apart from CO. Figure 3 shows examples of several PAH structures. Their reasonably large surface area can facilitate molecular synthesis. In the diffuse interstellar medium PAHs are supposed to be present in ionized form (as cations). No PAH molecule has been unambiguously identified yet. However, the infrared emission bands observed ubiquitously in the interstellar medium may represent families of PAH molecules (Peeters et al. 2002) with a range of size distribution (Bauschlicher et al. 2009), a number of different structures (Hony et al. 2001) and ionization states (Bauschlicher et al. 2009). Variations in the relative strength of infrared emission bands and variations in the peak position and profiles have contributed many suggestions of possible PAHs species as well as large species that may include heteroatoms and form clusters up to nm sized small carbonaceous grains (Peeters et al. 2002; Hudgins et al. 2005; Bauschlicher et al. 2008, 2009; Tielens 2008). Only small species up to 50 carbon atoms can be measured in the laboratory. The spectrum of larger PAHs can only be derived from theoretical calculations, such as density functional theory.
Figure 2.
Astronomical spectra of the infrared vibrational modes (at 3.3, 6.2, 7.7, 8.6, 11.2, and 12.7 µm) of PAH molecules in three objects that represent the different peak positions and relative intensities observed in various galactic and extragalactic regions (see Peeters et al. 2002). Classes A, B and C represent the three different astronomical environments; (A) ISM, reflection nebulae, HII regions; (B) a few post-AGB and Herbig Ae/Be stars and most Planetary Nebulae; (C) a few peculiar post-AGB stars.
Figure 3.
A few examples of PAH structures that might be present in the ISM.
Refractory Compounds
Carbon solids are ubiquitous material in interstellar space. However, the formation pathway of carbonaceous matter in astrophysical environments, as well as in terrestrial gas-phase condensation reactions, is not yet understood (Jaeger et al. 2008). Laboratory simulations in combination with interstellar observations support the idea that the predominant fraction of carbon in space is present as solid macromolecular carbon (e.g., Pendleton and Allamandola, 2002) or amorphous and hydrogenated amorphous carbon (Mennella et al. 1998; Duley and Lazarev 2004; Dartois et al. 2005). Fullerenes (Iglesias-Groth 2004) or defective carbon “onions” (Tomita et al. 2004) have also been proposed. Fullerenes of astronomical origin have been detected in meteorites and in and around an impact crater on the Long Duration Exposure Facility spacecraft; evidence for C60+ in the interstellar gas is provided by near infrared observations (Foing and Ehrenfreund 1996; see Ehrenfreund et al. 2006b for a review). Gas-phase condensation reactions in the laboratory demonstrate that the temperature in the condensation zone determines the formation pathway of carbonaceous particles. Condensation products in different astrophysical environments such as cool asymptotic giant branch stars or hot Wolf-Rayet stars should be different and should have distinct spectral properties (Jaeger et al. 2008).
Many of the described carbon compounds fail to match the observational constraints or account for the interstellar carbon budget (Snow and Witt 1995). The evolution of amorphous carbon measured by spectroscopy in the laboratory currently provides the best fit and satisfies the interstellar carbon balance of theoretical dust models (Menella et al. 1998). A combination of UV irradiated amorphous carbon (AC) and hydrogenated amorphous carbon (HAC) can explain the behavior of the bump structure (Mennella et al. 1998). Higher density regions display a larger width, compatible with hydrogenation of amorphous carbon. An alternative route to laboratory experiments at high temperature that simulate circumstellar environments is the production of hydrogenated carbon polymers through photolysis of a series of organics at low temperature (Dartois et al. 2005).
Interstellar Ices
Ground-based observations revealed already decades ago abundant water ice as well as CO (e.g. Whittet 1993). But other species remained undetected until infrared satellites opened up the 1–200 µm spectral window. Infrared Space Observatory (ISO) data were compiled to establish an inventory of interstellar ice species and measure their abundances in various interstellar environments (Gibb et al. 2004). After ISO, the Spitzer Space telescope (although operating at lower resolution) and ground-based observations have provided outstanding data on the main interstellar ice species. Recent c2d (core to disks) Spitzer surveys of ices investigated multiple sources, in particular low mass stars. Observations of the 6–8 µm region that displays the prominent bending mode of water ice shows five independent components that can be attributed to eight different carriers (Boogert et al. 2008). The spectrum is dominated by simple species formed by grain surface chemistry, which include CH3OH (1%–30%), NH3 (3%–8%), HCOOH (1%–5%), H2CO (∼6%), and HCOO− (0.3%) relative to water ice (Boogert et al. 2008). A comparison to high mass stars showed a rather similar ice distribution arguing against substantial UV radiation processing. CO2 ice has been identified by ISO as a ubiquitous and abundant ice species (Gerakines et al. 1999). The characteristic band profile of the CO2 bending mode at 15.2 µm has been used to identify the ice composition and characteristics in many lines of sight (Ehrenfreund et al. 1998, Boogert and Ehrenfreund 2004). Follow-up observations on a large sample of low mass stars during the c2d Spitzer survey showed higher abundances (average of 32% relative to water ice) (Pontoppidan et al. 2008). The survey also showed that CO2 is mixed in different ice compositions within the line of sight, namely into a water rich component and in apolar ice mixtures (containing CO). Pure CO2 ice layers were confirmed that indicate thermal processing actually described as “distillation” after CO has evaporated in cloud regions that exceed 20 K. A c2d Spitzer survey of CH4 ice showed that 25 out of 52 targets displayed a feature at 7.7 µm attributed to CH4. The abundances range from 2%–8% relative to water ice and reach 13% in a few sources (Oberg et al. 2008). The abundances are consistent with grain surface reaction formation of CH4. Ice abundances of CH4 seem to correlate with H2O and CO2 but not with CO and CH3OH. Photodesorption of pure ices seem to be much more efficient according to laboratory results than previously assumed (Öberg et al. 2009).
CHALLENGES
Stability of Organic Molecules
To understand what compounds can survive solar system formation or extraterrestrial delivery the thermal and radiation stability of organic compounds and biomolecules has to be investigated in laboratory studies. During the formation of our solar system, interstellar gas and dust were mixed, processed and partly destroyed according to their distance from the forming star. Radiation chemistry involving X-rays and UV light acted on upper disk layers (Gorti et al. 2009). Results from the Sun in Time program suggests that the coronal X-ray EUV emissions of the young main sequence Sun were 100–1000 times stronger than those of the present Sun (Ribas et al. 2005). At the estimated time period of the origin of life on Earth ∼3.5 billion years ago, the solar high energy UV flux was 6 times the present value. The strong radiation emissions inferred, together with geological processes, may provide limits to the survival of organic compounds and biochemical pathways to create life.
Recent laboratory studies monitored the photostability of small N-heterocycles, nucleobases, benzene, and ethers using matrix-isolation spectroscopy at low temperature (Peeters et al. 2003, 2005, 2006). Amino acids have a limited life span in the interstellar medium (Ehrenfreund et al. 2001; Peeters et al. 2003). Amino acid photolysis under Martian conditions has been investigated by ten Kate et al. (2005) and showed a similar result, namely half lives of less than 22 h for glycine on the Martian surface. N-heterocycles are more easily destroyed than their carbonaceous cognate molecules such as benzene (Peeters et al. 2005). Furthermore, heterocycles containing several N-atoms in the ring, such as adenine have dramatically decreased half-lives when exposed to UV radiation.
In the gas phase, even benzene and small PAHs are not stable enough to survive the strong radiation fields in the diffuse interstellar medium (Ruiterkamp et al. 2005b, Peeters et al. 2005). Thin film radiation studies show that coronene and perylene, both pericondensed PAHs, are four to six times more stable than fluoranthene (which contains a pentagon ring) and 2,3-benzanthracene, respectively (Ehrenfreund et al. 2007). In comparison, the destruction of a thin film of the amino acid d-alanine measured under the same conditions proceeded ∼50 times faster than the PAH coronene. Detailed analyses of astronomical observations indicate sizes for PAHs of at least 50 carbon atoms and up to several hundred carbon atoms (Bauschlicher et al. 2009) (see Fig. 3).
Prebiotic Molecules in Space—Any Relevance for Early Earth
Aromatic molecules and biomarkers are widespread in our galaxy and beyond (Ehrenfreund et al. 2006a). The investigation of their life cycle is highly relevant for cosmochemistry. Molecules that are important in biochemical pathways such as nitriles, aldehydes, ethers, ketones, amines, and amides have been observed in space (www.astrochemistry.net). Even the amino acid glycine has been identified in hot molecular cores (Kuan et al. 2003).
However, as discussed earlier, most of those biomolecules are neither radiation nor thermal-resistant species, and are easily destroyed by UV radiation, shocks and thermal processing. Consequently they are unlikely to survive incorporation into solar system material without some degradation (see Stability of Organic Molecules above). A link between prebiotic compounds identified in short-lived hot core regions to the origin of life on Earth is therefore not realistic.
Prebiotically significant organic material identified in meteorites is formed within the solar system in radiation shielded environments and in the presence of liquid water. This is strongly supported by recent results that provide evidence that the organic composition in carbonaceous meteorites is dependent on parent body alteration processes, in particular aqueous alteration (Martins et al. 2007; Glavin and Dworkin 2009).
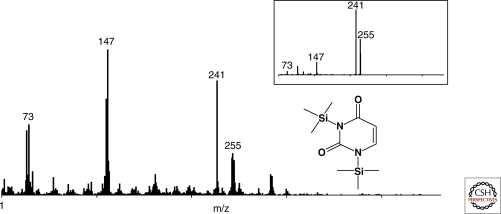
In addition to amino acids and sugar-related compounds, another important variety of precursor molecules, namely nucleobases, were recently detected in the Murchison meteorite (Martins et al. 2008). Compound-specific carbon isotope data of measured purine and pyrimidine compounds indicated a nonterrestrial origin for these compounds (see Fig. 4). Consequently, nucleobases delivered to these worlds together with sugar- related species and amino acids might have been beneficial to the origin of life on Earth, Mars, or elsewhere. The high abundance of aromatic material in meteorites led to the hypothesis of the “aromatic world” that describes PAH-based transitions from non-living to living matter as an interesting alternative to traditional origin of life models (Ehrenfreund et al. 2006a). Observations of cometary comae indicate that comets are rich in organic compounds and will have effectively delivered carbonaceous solids and volatiles to the surface of young planets. In contrast, the endogenous synthesis of prebiotic organic compounds may have been constrained by the conditions on the young Earth. Whatever the inventory of endogenous organic compounds on the ancient Earth, it would have been augmented by extraterrestrial material. It is estimated that these sources delivered ∼109kg of carbon per year to the Earth during the heavy bombardment phase 4.5–3.9 billion years ago (Chyba and Sagan 1992).
Figure 4.
The gas chromatography/mass spectrometer spectrum for the peak assigned to BSTFA (N,o-Bis Trimethylsilyl (trifluoroacetamide) derivatization reagent) derivatized uracil and its structure. The inset shows the mass spectrum of a BSTFA-derivatized uracil standard (Martins et al. 2008).
RESEARCH DIRECTIONS AND CONCLUDING REMARKS
Several compounds are observed ubiquitously in our and external galaxies. Among them are PAHs, aliphatic hydrocarbons and ices (see Pendleton and Allamandola 2002; Ehrenfreund et al. 2006a; Tielens 2008 for reviews). The so-called “ultraviolet extinction bump” (at 2175Å) in the interstellar extinction curve is attributed to solid carbon-bearing material. The position of this feature attributed to amorphous carbon compounds is practically invariant in galactic and extragalactic regions, which argues for an abundant and stable carrier. To respond to current challenges described in Section 4, the following future research directions are proposed: (1) Investigation of the link between presolar and solar system material, (2) Revealing formation mechanisms/sites for macromolecular carbon networks, (3) Identification of specific PAHs, (4) Defining the role of small bodies in extraterrestrial delivery processes.
Understanding the implications of extraterrestrial delivery requires substantial knowledge of planetary dynamics, disk and solar nebula chemistry. There is a huge difference between making or discovering amino acids in space and creating life and the origin of life and the survival of existing life through adaptation in apparently impossible environments. It is questionable if the most abundant organic material, namely aromatic hydrocarbons and carbonaceous networks are formed only in circumstellar environments. Exploring mechanisms to form aromatics in the low temperature diffuse interstellar medium represent an important research avenue. The infrared emission bands that have identified aromatic compounds in galactic and extragalactic regions sample multi-cloud lines of sight and consequently a size and shape and likely ionization state distribution of PAHs. The identification of individual PAH molecules through their vibrational transitions will be very difficult. Only a combination of observations, laboratory data and theoretical calculations will provide constraints for the identification of a specific PAH class or family. Small bodies, such as comets, asteroids and their fragments, meteorites and interplanetary dust particles (IDPs) bear witness of processes occurring at the time of solar system formation. Astronomical observations, improved technology for extraterrestrial sample analysis, in-situ surface measurements of small solar system bodies and/or returned samples will strongly enhance the knowledge of the role of small bodies in impact and extraterrestrial delivery processes to young planets. These research avenues will also guide us to extend our knowledge to other habitable worlds.
ACKNOWLEDGMENTS
PE is supported by NASA grant NNX08AG78G and the NASA Astrobiology Institute NAI.
Editors: David Deamer and Jack W. Szostak
Additional Perspectives on The Origins of Life available at www.cshperspectives.org
REFERENCES
- Alexander CMO'D, Fogel M, Yabuta H, Cody GD 2007. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim Cosmochim Acta 71: 4380–4403 [Google Scholar]
- Allamandola LJ, Hudgins DM, Sandford SA 1999. Modeling the unidentified infrared emission with combinations of polycyclic aromatic hydrocarbons. ApJ 511: 115–119 [DOI] [PubMed] [Google Scholar]
- Bauschlicher CW, Peeters E, Allamandola LJ 2008. The infrared spectra of very large, compact, highly symmetric, polycyclic aromatic hydrocarbons (PAHs). ApJ 678: 316–327 [Google Scholar]
- Bauschlicher CW, Peeters E, Allamandola LJ 2009. the infrared spectra of very large irregular polycyclic aromatic hydrocarbons (PAHs): Observational probes of astronomical PAH geometry, size, and charge. ApJ 697: 311–327 [Google Scholar]
- Bertoldi F, Carilli CL, Cox P, Fan X, Strauss MA, Beelen A, Omont A, Zylka R 2003. Dust emission from the most distant quasars. Astronomy and Astrophysics 406: L55–L58 [Google Scholar]
- Blum J 2004. Grain growth and coagulation. In Astrophysics of Dust (eds Witt A.N., Clayton G.C., and Draine B.T.) Vol. 309, pp. 369–392 ASP Conference Series [Google Scholar]
- Boogert ACA, Pontoppidan KM, Knez C, Lahuis F, Kessler-Silacci J, van Dishoeck EF, Blake GA, Augereau J-C, Bisschop SE, Bottinelli S, et al. 2008. The c2d Spitzer Spectroscopic Survey of Ices around Low-Mass Young Stellar Objects. I. H2O and the 5–8 µm Bands. ApJ 678: 985–1004 [Google Scholar]
- Boogert ACA, Ehrenfreund P 2004. Interstellar Ices. In Astrophysics of Dust (eds Witt A.N., Clayton G.C., and Draine B.T.) Vol. 309, pp. 547 ASP Conference Series [Google Scholar]
- Boss AP 2004. From molecular clouds to circumstellar disks. In: COMETS II, (eds Festou M.C., Keller H.U., and Weaver H.A.) Univ. Arizona Press, 67–80 [Google Scholar]
- Brownlee D, Tsou P, Aléon J, Alexander CMOD, Araki T, Bajt S, Baratta GA, Bastien R, Bland P, Bleuet P, et al. 2006. Comet 81P/Wild 2 Under a Microscope. Science 314: 1711–1716 [DOI] [PubMed] [Google Scholar]
- Cataldo F 2004. From Elemental Carbon to Complex Macromolecular Networks in Space. In Astrobiology: Future Perspectives (eds Ehrenfreund P., Becker L., and Blank J.) Astrophysics and Space Science Library, Vol. 305, pp. 97–126 Kluwer Academic Publishers: Dordrecht, The Netherlands [Google Scholar]
- Charnley SB, Ehrenfreund P, Kuan Y 2003. Molecules in Space, Physics World, Institute of Physics Publishing Ltd, October 2003, 35–38 [Google Scholar]
- Charnley SB, Kuan Y, Huang H, Botta O, Butner HM, Cox N, Despois D, Ehrenfreund P, Kisiel Z, Lee Y, et al. 2005. Astronomical searches for nitrogen heterocycles. Adv Space Res 36: 137–145 [Google Scholar]
- Chiar JE, Pendleton Y 2008. The origin and evolution of interstellar organics. Organic Matter in Space. Proceedings of the International Astronomical Union, IAU Symposium, Volume 251: 35–44 [Google Scholar]
- Chiar JE, Ennico K, Pendleton YJ, Boogert ACA, Greene T, Knez C, Lada C, Roellig T, Tielens AGGM, Werner M, et al. 2007. The relationship between the optical depth of the 9.7 µm silicate absorption feature and infrared differential extinction in dense clouds. ApJ 666: L73–L76 [Google Scholar]
- Chick K, Cassen P 1997. Thermal Processing of Interstellar Dust Grains in the Primitive Solar Environment. ApJ 477: 398–409 [Google Scholar]
- Chyba C, Thomas P, Brookshaw L, Sagan C 1990. cometary delivery of organic molecules to the early Earth. Science 249: 366–373 [DOI] [PubMed] [Google Scholar]
- Chyba C, Sagan C 1992. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 355: 125–132 [DOI] [PubMed] [Google Scholar]
- Ciesla F 2009. Observing our origins. Science 319: 1488–1489 [DOI] [PubMed] [Google Scholar]
- Cottin H, Fray N 2008. Distributed Sources in Comets. Space Sci Rev 138: 179–197 [Google Scholar]
- Cronin JR, Chang S 1993. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorite. In The chemistry of life's origin (eds Greenberg J.M., Mendoza-Gomez C.X., Pirronello V.) pp. 209–258 Kluwer Academic Publishing, Dordrecht, The Netherlands [Google Scholar]
- Crovisier J, Biver N, Bockelée-Morvan D, Boissier J, Colom P, Dariusz C 2009. The chemical diversity of comets: synergies between space exploration and ground-based radio observations. Earth, Moon, and Planets 105: 267–272 [Google Scholar]
- Cruikshank D, Imanaka H, Dalle O, Cristina M 2005. Tholins as coloring agents on outer Solar System bodies. Adv Space Res 36: 178–183 [Google Scholar]
- Dartois E, Muñoz Caro GM, Deboffle D, Montagnac G, D'Hendecourt L 2005. Ultraviolet photoproduction of ISM dust. Laboratory characterisation and astrophysical relevance. Astronomy and Astrophysics 432: 895–908 [Google Scholar]
- Derenne S, Robert F, Skrzypczak-Bonduelle A, Gourier A, Binet L, Rouzaud JN 2008. Molecular evidence for life in the 3.5 billion year old Warrawoona Chert. Earth and Planetary Sci Lett 272: 476–480 [Google Scholar]
- Di Santi M, Mumma M 2009. Reservoirs for comets: compositional differences based on infrared observations, origin and early evolution of comet nuclei. Space Sciences Series28: 127 [Google Scholar]
- Duley WW, Lazarev S 2004. Ultraviolet absorption in amorphous carbons: Polycyclic aromatic hydrocarbons and the 2175 Å extinction feature. ApJ612: L33–L35 [Google Scholar]
- Dullemond C, Pavlyuchenkov Y, Apai D, Pontoppidan K 2008. Structure and evolution of protoplanetary disks. In Structure and evolution of protoplanetary disks, Phys Conf Ser 131: 012018 [Google Scholar]
- Ehrenfreund P, Dartois E, Demyk K, d'Hendecourt L 1998. Ice segregation toward massive protostars. AstronomyAstrophysics Lett 339: L17–L21 [Google Scholar]
- Ehrenfreund P, Charnley SB 2000. Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to the early Earth. Ann Rev Astron Astrophys 38: 427–483 [Google Scholar]
- Ehrenfreund P, Glavin D, Botta O, Cooper G, Bada J 2001. Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of CI type carbonaceous chondrites. PNAS Special Issue on Astrobiology98: 2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfreund P, Irvine W, Becker L, Blank J, Brucato JR, Colangeli L, Derenne S, Despois D, Dutrey A, Fraaije H, et al. 2002. Astrophysical and astrochemical insights into the origin of life. Reports on Progress in Physics 65: 1427–1487 [Google Scholar]
- Ehrenfreund P, Fraser H 2003. Ice chemistry in space. : Solid state Astrochemistry, NATO ASI Series, (eds Pirronello V., Krelowski K., and Manicò G.) pp. 317–356 Kluwer Academic Publishers [Google Scholar]
- Ehrenfreund P, Charnley SB, Wooden DH 2004. From ISM material to comet particles and molecules. In COMETS II, (eds Festou M.C., Keller H.U., and Weaver H.A.), pp 115–133 Univ. Arizona Press [Google Scholar]
- Ehrenfreund P, Rasmussen S, Cleaves JH, Chen L 2006aExperimentally tracing the key steps in the origin of life: The aromatic world. Astrobiology 6/3: 490–520 [DOI] [PubMed] [Google Scholar]
- Ehrenfreund P, Cox N, Foing BH 2006b. Fullerenes and related carbon structures in stellar atmospheres and the interstellar medium. In: Natural Fullerenes and related structures of elemental carbon. (ed. Rietmeijer F.) Series Developments in Fullerene Science, Vol. 6, 57–63 [Google Scholar]
- Ehrenfreund P, Ruiterkamp R, Peeters Z, Foing B, Salama F, Martins Z 2007. The ORGANICS experiments on BIOPAN V: UV and space exposure of aromatic compounds. Planetary Space Sci 55: 383–400 [Google Scholar]
- Foing BH, Ehrenfreund P 1994. Detection of two interstellar absorption bands coincident with spectral features of C60+. Nature 369: 296–298 [Google Scholar]
- Genzel R, Lutz D, Sturm E, Egami E, Kunze D, Moorwood AFM, Rigopoulou D, Spoon HWW, Sternberg A, Tacconi-Garman LE, et al. 1998. What powers ultraluminous IRAS Galaxies? Astrophysical J 498: 579 [Google Scholar]
- Gerakines PA, Whittet DCB, Ehrenfreund P, Boogert ACA, Tielens AGGM, Schutte WA, Chiar JE, van Dishoeck EF, Prusti T, Helmich FP, et al. 1999. ISO-SWS observations of solid carbon dioxide in molecular clouds. Astrophysical J 522: 357 [Google Scholar]
- Gibb E, Whittet D, Boogert A, Tielens AGGM 2004. Interstellar ice: The infrared space observatory legacy. Astrophysical J Supp 151: 35–73 [Google Scholar]
- Glavin DP, Dworkin JP 2009. Enrichment in L-Isovaline by Aqueous Alteration on CI and CM Meteorite Parent Bodies. PNAS 106/14: 5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R, Levison HF, Tsiganis K, Morbidelli A 2005. Origin of the cataclysmic Late Heavy Bombardment period of the terrestrial planets. Nature 435: 466–469 [DOI] [PubMed] [Google Scholar]
- Gorti U, Dullemond CP, Hollenbach D 2009. Time Evolution of Viscous Circumstellar Disks due to Photoevaporation by Far-Ultraviolet, Extreme-Ultraviolet, and X-ray Radiation from the Central Star. Astrophysical J 705: 1237–1251 [Google Scholar]
- Greenberg J 1998Making a comet nucleus. Astronomy and Astrophysics 330: 375–380 [Google Scholar]
- Henning T, Mutschke H 2004. In Astrophysics of Dust (eds Witt A.N., Clayton G.C., and Draine B.T.) Vol. 309, p. 603, ASP Conference Series [Google Scholar]
- Henning T, Salama F 1998. Carbon in the Universe. Science 282: 2204–2210 [DOI] [PubMed] [Google Scholar]
- Hiroi T, Pieters C, Zolensky ME, Lipschutz ME 1993. Evidence of thermal metamorphism on the C, G, B, and F asteroids. Science 261: 1016–1018 [DOI] [PubMed] [Google Scholar]
- Hony S, Van Kerckhoven C, Peeters E, Tielens AGGM, Hudgins DM, Allamandola LJ 2001. The CH out-of-plane bending modes of PAH molecules in astrophysical environments. Astronomy and Astrophysics 370: 1030–1043 [Google Scholar]
- Hudgins DM, Bauschlicher CW, Allamandola LJ 2005Variations in the peak position of the 6.2 µm interstellar emission feature: A tracer of n in the interstellar polycyclic aromatic hydrocarbon population. Astrophysical J 632: 316–332 [Google Scholar]
- Iglesias-Groth S 2004. Fullerenes and buckyonions in the interstellar medium. Astrophysical J 608: L37–L40 [Google Scholar]
- Jäger C, Mutschke H, Henning Th, Huisken F 2008. Spectral properties of gas-phase condensed fullerene-like carbon nanoparticles from far-ultraviolet to infrared wavelengths. ApJ689: 249–259 [Google Scholar]
- Kuan Y, Charnley S, Huang H, Tseng W, Kisiel Z 2003. Interstellar glycine. Astrophysical J 593: 848–867 [Google Scholar]
- Kwok S 2004. The synthesis of organic and inorganic compounds in evolved stars. Nature 430: 985–991 [DOI] [PubMed] [Google Scholar]
- Kwok S 2009. Delivery of complex organic compounds from planetary nebulae to the solar system. Intern. J Astrobiol 8/3: 161–167 [Google Scholar]
- Lammer H, Bredehöft JH, Coustenis A, Khodachenko ML, Kaltenegger L, Grasset O, Prieur D, Raulin F, Ehrenfreund P, Yamauchi M, et al. 2009. What makes a planet habitable? Astron Astrophys Rev 17: 181–249 [Google Scholar]
- Liszt H, Lucas R 2000. The structure and stability of interstellar molecular absorption line profiles at radio frequencies. Astronomy and Astrophysics 355: 333–346 [Google Scholar]
- Lorenz RD, Mitchell KL, Kirk RL, Hayes AG, Aharonson O, Zebker HA, Paillou P, Radebaugh J, Lunine JI, Janssen MA, et al. 2008. Titan's inventory of organic surface materials. Geophys Res Lett 35: L02206 [Google Scholar]
- Markwick A, Charnley SB 2004. Chemistry of Protoplanetary Disks. In Astrobiology: Future Perspectives (eds Ehrenfreund P., Becker L., and Blank J.) Astrophysics and Space Science Library, Vol. 305, pp. 33–66 Kluwer Academic Publishers: Dordrecht, The Netherlands [Google Scholar]
- Martins Z, Alexander OD, Orzechowska G, Fogel M, Ehrenfreund P 2007. Indigenous amino acids and chiral excess identified in CR primitive meteorites. Meteoritics and Planetary Sci 42/12: 2125–2136 [Google Scholar]
- Martins Z, Botta O, Fogel ML, Sephton MA, Glavin DP, Watson JS, Dworkin JP, Schwartz AW, Ehrenfreund P 2008. Extraterrestrial nucleobases in the Murchison meteorite. Earth and Planetary Sci Lett 270: 130–136 [Google Scholar]
- Mathis J, Mezger P, Panagia N 1983. Interstellar radiation field and dust temperatures in the diffuse interstellar matter and in giant molecular clouds. Astronomy and Astrophysics 128: 212–229 [Google Scholar]
- Mennella V, Colangeli L, Bussoletti E, Palumbo P, Rotundi A 1998. A new approach to the puzzle of the ultraviolet interstellar extinction bump. Astrophysical Jl 507: 177–180 [Google Scholar]
- Mullie F, Reisse J 1987. Organic Matter in carbonaceous chondrites. Topics in Current Chemistry 139: 83 [Google Scholar]
- Nesvorny D, Jenniskens P, Levison HF, Bottke WF, Vokrouhlicky D 2009. Cometary origin of the zodiacal cloud and carbonaceous micrometeorites. Astrophysical J, in press [Google Scholar]
- Öberg KI, Boogert ACA, Pontoppidan KM, Blake GA, Evans NJ, Lahuis F, van Dishoeck EF 2008. The c2d spitzer spectroscopic survey of ices around low-mass young stellar objects. III. CH4. Astrophysical J 678: 1032–1041 [Google Scholar]
- Öberg K, Bottinelli S, van Dishoeck EF 2009. Cold gas as an ice diagnostic toward low mass protostars. Astronomy and Astrophysics 494: L13–L16 [Google Scholar]
- Peeters E, Hony S, van Kerckhoven C, Tielens AGGM, Allamandola L, Hudgins DM, Bauschlicher CW 2002. The rich 6 to 9 µm spectrum of interstellar PAHs. Astronomy and Astrophysics 390: 1089–1113 [Google Scholar]
- Peeters E, Allamandola LJ, Hudgins DM, Hony S, Tielens AGGM 2004a. The unidentified infrared features after ISO. In Astrophysics of Dust (eds Witt A.N., Clayton G.C., and Draine B.T.) 309: 141 ASP Conference Series [Google Scholar]
- Peeters E, Spoon HWW, Tielens AGGM 2004b. polycyclic aromatic hydrocarbons as a tracer of star formation? Astrophysical J 613: 986–1003 [Google Scholar]
- Peeters Z, Botta O, Ruiterkamp R, Charnley SB, Ehrenfreund P 2003. The astrobiology of nucleobases. Astrophysical J Lett 593: L129–L132 [Google Scholar]
- Peeters Z, Botta O, Charnley SB, Kuan YL, Kisiel Z, Ehrenfreund P 2005. Formation and photostability of N-heterocycles in space: The effect of nitrogen on the photostability of small aromatic molecules. Astronomy and Astrophysics 433: 583–590 [Google Scholar]
- Peeters Z, Rodgers S, Charnley S, Schriver A, Schriver-Mazzuoli L, Keane J, Ehrenfreund P 2006. Astrochemistry of dimethyl ether. Astronomy and Astrophysics 445: 197 [Google Scholar]
- Pendleton YJ, Allamandola LJ 2002. The organic refractory material in the diffuse interstellar medium: mid-infrared spectroscopic constraints. Astrophysical J Supplement 138: 75–98 [Google Scholar]
- Pontoppidan KM, Boogert ACA, Fraser HJ, van Dishoeck EF, Blake GA, Lahuis F, Öberg KI, Evans J, Salyk C 2008. The c2d spitzer spectroscopic survey of ices around low-mass young stellar objects. II. CO2. Astrophysical J 678: 1005–1031 [Google Scholar]
- Prasad S, Tarafdar SP 1983. UV radiation field inside dense clouds - Its possible existence and chemical implications. Astrophysical J 267: 603–609 [Google Scholar]
- Quinn R, Zent A, Grunthaner F, Ehrenfreund P, Taylor C, Garry J 2005. Detection and characterization of oxidizing acids in the Atacama desert using the Mars Oxidation Instrument. Planetary Space Sci 53: 1376–1388 [Google Scholar]
- Raulin F 2008. Astrobiology and habitability of Titan. Space Sci Rev 135: 37–48 [Google Scholar]
- Ribas I, Guinan E, Güdel M, Audard M 2005. Evolution of the solar activity over time and effects on planetary atmospheres. I. High-Energy irradiances (1–1700 Å). Astrophysical J 622: 680–694 [Google Scholar]
- Ruiterkamp R, Cox N, Spaans M, Kaper L, Salama F, Foing B, Ehrenfreund P 2005a. The PAH charge state distribution in diffuse and translucent clouds: Implications for DIB carriers. Astronomy and Astrophysics 432: 515 [Google Scholar]
- Ruiterkamp R, Peeters Z, Moore M, Hudson R, Ehrenfreund P 2005b. A quantitative study of proton irradiation and UV photolysis of benzene in interstellar environments. Astronomy and Astrophysics 440: 391 [Google Scholar]
- Salama F 1999. Polycyclic aromatic hydrocarbons in the interstellar medium. In Solid Interstellar Matter - The ISO Revolution (eds d'Hendecourt L., Jones A., and Joblin C.) p. 65, EDP Sciences and Springer-Verlag [Google Scholar]
- Sephton MA 2002. Organic compounds in carbonaceous meteorites. Nat Prod Rep 19: 292–311 [DOI] [PubMed] [Google Scholar]
- Sephton MA, Botta O 2005. Recognizing life in the solar system: guidance from meteoritic organic matter. Int J Astrobiol 4: 269–276 [Google Scholar]
- Smith JDT, Draine BT, Dale DA, Moustakas J, Kennicutt RC Jr, Helou G, Armus L, Roussel H, Sheth K, Bendo GJ 2007. The mid-infrared spectrum of star-forming galaxies: global properties of polycyclic aromatic hydrocarbon emission. Astrophysical J 656: 770–791 [Google Scholar]
- Snow T, Witt A 1995. The interstellar carbon budget and the role of carbon in dust and large molecules. Science 270: 1455–1460 [DOI] [PubMed] [Google Scholar]
- Snow TP, McCall BJ 2006. Diffuse atomic and molecular clouds. Ann Rev Astronomy Astrophys 44: 367–414 [Google Scholar]
- Spaans M 2004. The synthesis of the elements and the formation of stars. In Astrobiology: Future Perspectives (eds Ehrenfreund P., Becker L., and Blank J.) Astrophysics and Space Science Library, Vol. 305, pp. 1–16 Kluwer Academic Publishers: Dordrecht, The Netherlands [Google Scholar]
- ten Kate I, Garry J, Peeters Z, Quinn R, Foing BH, Ehrenfreund P 2005. Amino acid photostability on the Martian surface. Meteoritics and Planetary Science 40: 1185–1193 [Google Scholar]
- Tielens AGGM 2008. Interstellar polycyclic aromatic hydrocarbon molecules. Ann Rev of Astronomy and Astrophysics 46: 289–337 [Google Scholar]
- Tomita S, Fujii M, Hayashi S 2004. Defective carbon onions in interstellar space as the origin of the optical extinction bump at 217.5 nanometers. ApJ609: 220–224 [Google Scholar]
- Van Dishoeck EF, Blake G 1998. Chemical evolution of star-forming regions. Ann Rev of Astronomy and Astrophysics 36: 317. [DOI] [PubMed] [Google Scholar]
- Van Dishoeck EF 2004. ISO Spectroscopy of gas and dust: from molecular clouds to protoplanetary disks. Ann Review of Astronomy and Astrophysics 42: 119–167 [Google Scholar]
- Visser R, Geers VC, Dullemond CP, Augereau J, Pontoppidan K, van Dishoeck EF 2007. PAH chemistry and IR emission from circumstellar disks. Astronomy and Astrophysics 466: 229–241 [Google Scholar]
- Whittet D 1993. Observations of Molecular Ices. Dust and Chemistry in Astronomy. The Graduate Series in Astronomy Dust (eds Millar T.J. and Williams D.A.) Institute of Physics Publishing, PA, pp. 9 Philadelphia [Google Scholar]
- Wolfire MG, McKee CF, Hollenbach D, Tielens AGGM 2003. Neutral atomic phases of the interstellar medium in the galaxy. ApJ 587: 278–311 [Google Scholar]
- Wooden DH, Charnley SB, Ehrenfreund P 2004. Composition and evolution of molecular clouds. In: COMETS II (eds Festou M.C., Keller H.U., and Weaver H.A.) Univ. Arizona Press, 33–66 [Google Scholar]
- Yan L, Chary R, Armus L, Teplitz H, Helou G, Frayer D, Fadda D, Surace J, Choi P 2005. Spitzer detection of polycyclic aromatic hydrocarbon and silicate dust features in the mid-infrared spectra of z ∼2 ultraluminous infrared galaxies. ApJ628: 604–610 [Google Scholar]