Abstract
Cheating is common in cooperative interactions, but its occurrence can be controlled by various means ranging from rewarding cooperators to active punishment of cheaters. Punishment occurs in the mutualism involving the cleanerfish Labroides dimidiatus and its reef fish clients. When L. dimidiatus cheats, by taking scales and mucus rather than ectoparasites, wronged clients either chase or withhold further visits to the dishonest cleaner, which leads to more cooperative future interactions. Punishment of cheating L. dimidiatus may be effective largely because these cleaners are strictly site-attached, increasing the potential for repeated interactions between individual cleaners and clients. Here, we contrast the patterns of cheating and punishment in L. dimidiatus with its close relative, the less site-attached Labroides bicolor. Overall, L. bicolor had larger home ranges, cheated more often and, contrary to our prediction, were punished by cheated clients as frequently as, and not less often than, L. dimidiatus. However, adult L. bicolor, which had the largest home ranges, did not cheat more than younger conspecifics, suggesting that roaming, and hence the frequency of repeated interactions, has little influence on cheating and retaliation in cleaner–client relationships. We suggest that roaming cleaners offer the only option available to many site-attached reef fish seeking a cleaning service. This asymmetry in scope for partner choice encourages dishonesty by the partner with more options (i.e. L. bicolor), but to be cleaned by a cleaner that sometimes cheats may be a better option than not to be cleaned at all.
Keywords: conflict, cooperation, biological markets, cheating, partner switching, territory size
1. Introduction
The potential for conflict is common in cooperative interactions. This counterintuitive situation arises because in many cooperative interactions, the interests of partners are not perfectly aligned. The strong selection on each partner to attempt to derive the maximum net benefits from the interaction (Trivers 1971; Axelrod & Hamilton 1981; Pellmyr & Huth 1994; Connor 1995) may thus lead to ‘cheating’ by one or both of the parties. Cheating occurs when either of the cooperative partners deceives the other by providing a dishonest service, which can range from a subtle reduction in service to not delivering the expected benefit at all (Caullery 1952; McDade & Kinsman 1980; Bronstein 1991; Mooring & Mundy 1996; Yu & Pierce 1998; Bshary 2002a). This functional definition implies that the deceived partner usually incurs a cost from interacting with a cheater rather than with an honest partner (Semple & McComb 1996).
Cheating has long been predicted to lead to instability and break-down of mutualistic interactions (Caullery 1952; Trivers 1971; Axelrod & Hamilton 1981). However, this prediction has not been borne out, largely because a variety of means for controlling cheating or conflict resolution have evolved in symbiotic organisms. Strategies include simply reducing the opportunity for partners to cheat, rewarding cooperators while applying sanctions to cheaters and active punishment of uncooperative individuals (reviewed in Douglas 2008). Sanctions involve a curtailment of investment by the cheated partner, and the benefit gained from such an action does not depend on the partner's future behaviour (Bergmuller et al. 2007). Examples include selective abortion of yucca fruits that contain too many pollinating moth larvae (Pellmyr 2002), selective reduction in legume nodule maintenance/growth if the rhizobia inhabitants do not fix enough nitrogen (Kiers et al. 2003) and withholding carbon rewards to arbuscular mycorrhizal fungi from plants as a function of phosphate provided (Fitter 2006). In all cases, the sanction is a side product of the plants' immediate resource-saving decisions (Bronstein 2001). Punishment, on the other hand, does not convey immediate benefits to the actor; the benefits are delayed and realized only after changes in the recipient's behaviour in response to the punishment (Clutton-Brock & Parker 1995). Punishment, as a mechanism to control cheating, depends on repeated interactions, learning and memory in the interacting partners.
There are few unambiguous examples of punishment. One of the most convincing ones occurs in the mutualism involving the bluestreak cleaner wrasse Labroides dimidiatus and its reef fish clients (Bshary & Würth 2001; Bshary & Grutter 2002b, 2005). Fish clients visit these cleanerfish at permanent locations, known as cleaning stations, to have their ectoparasites removed (reviewed by Côté 2000). However, bluestreak cleaner wrasses take not only parasites but also occasionally remove scales, mucus and healthy tissue from their clients (Grutter 1997; Bshary 2002a). In laboratory experiments, L. dimidiatus preferred client mucus over ectoparasites (Grutter & Bshary 2003, 2004), suggesting a direct conflict between the preferences of cleaners and clients. However, clients can punish cleaners that cheat (i.e. those that remove items other than ectoparasites), either by withholding further visits to a cheating cleaner if clients have several cleaners to choose from (non-resident clients) (Bshary & Schäffer 2002) or aggressive chasing, if clients have access to a single cleaning station (resident clients) (Bshary & Grutter 2002a). In the laboratory, both forms of punishment resulted in cleaners behaving honestly, i.e. feeding on ectoparasites rather than on mucus, in subsequent interactions (Bshary & Grutter 2005).
Punishment of cheating bluestreak cleaner wrasses by clients may be effective partly because these cleaners are strictly site-attached (Bshary 2002b). Fidelity of cleaners to a cleaning station increases both the potential for repeated interactions between individual cleaners and clients, as well as the potential for clients to recall the spatial location of a negative interaction, if not the exact identity of a cheating cleaner. Indeed, it has been argued that the mobility of partners should be a general hindrance to the evolution of cooperation (Dugatkin & Wilson 1991; Enquist & Leimar 1993).
Here, we examine the potential consequences of lack of site fidelity for cheating and punishment in a cleanerfish. The bicolor cleaner wrasse Labroides bicolor is a congener of the better known L. dimidiatus. Both species are similar in foraging mode; however, bicolor cleaner wrasses operate not from the limited area of a cleaning station, as L. dimidiatus does, but from large home ranges (Randall 2005). We predicted that this key difference in home range might be associated with two further differences: first, a reduced value to cleaners of individual cleaning interactions because of increased opportunities to encounter more clients, and second, a reduced scope for repeated interactions with individual clients. These effects should in turn be associated with differences in the extent of cheating by different cleanerfish species and punishment by their clients. Specifically, we expected that roaming L. bicolor should cheat more frequently than site-faithful L. dimidiatus and that owing to the lower likelihood of repeated interactions, punishment should be less frequently applied by the cheated clients of adult L. bicolor than by those of adult L. dimidiatus. The extent of cheating by cleaners should also increase with age and body size, if home range size is linked to these features. We tested these predictions, and the effects of clientele composition, through detailed field observations of both L. bicolor and L. dimidiatus at the same coral reef site.
2. Material and methods
(a). Behavioural observations
The study was carried out in the lagoon of Rangiroa Atoll (14°58′8 S, 147°38′3 W), in the Tuamotu Archipelago, French Polynesia, in March 2008. The study site was Motu Nuhi Nuhi, a large (10 000 m2) area of continuous and patch reef (locally referred to as ‘The Aquarium’) on the lagoon side of Tiputa Pass. Depth varied from 1 to 7 m and the substratum had high relief and high coral cover.
Observations of bluestreak and bicolor cleaner wrasses were carried out between 09.30 and 16.00 by snorkelling. Individual cleaners were selected haphazardly, and observations began upon sighting a new cleaner. On each observation day, a different section of the reef was searched to minimize the risk of repeat observations of cleaners. Observations were made from a distance of 2–3 m. Each individual cleaner was observed for 30 min, during which we recorded the number and species of each client interacting with the focal cleaner, whether the client jolted and the client's reaction following a jolt (i.e. chase or no chase). Jolts are apparently painful reactions by clients to a cleanerfish bite. They have previously been shown to be unrelated to the removal of ectoparasites and are considered to be indicators of dishonest biting by cleaners (Bshary & Grutter 2002a; Soares et al. 2008). At the end of each focal observation period, the maximum distance travelled by the cleaner (estimated visually) during the observation was noted, and used as a proxy for home range size. The total length of the focal cleaner was also estimated visually. Cleanerfish age was classified as juvenile, sub-adult or adult on the basis of coloration (Potts 1973).
(b). Statistical analysis
We first tested the assumptions of differences between cleaners in home range sizes and association between body length (or age) and home range size. Home range sizes were estimated from the maximum distance travelled by the cleaner during each focal observation and were log-transformed to attain normality. We used a two-way analysis of variance (ANOVA) to examine the effects of age (fixed factor: juvenile, sub-adult, adult) and cleanerfish species (random factor: L. dimidiatus and L. bicolor) on home range sizes. Simple-effects analyses (MANOVA) were used to break down the interaction term, by analysing the effect of one independent variable (e.g. species) at individual levels of the other independent variable (e.g. age). Syntax in SPSS (v. 17) was used to perform these analyses (Field 2005).
From the focal observations, we extracted measures of cheating and punishment behaviour. We tallied the number of clients inspected in 30 min for each cleanerfish. The extent of cheating by cleaners was then measured as the proportion of inspected clients that jolted. The prevalence of punishment by clients was measured as the proportion of jolting clients that chased each attending cleaner. Extent of cheating and prevalence of punishment were calculated for all clients combined, and then for two specific classes of clients that might be expected to vary in their response to cheating: residents (i.e. species with access to only one cleaning station) and non-residents (i.e. species with access to multiple cleaning stations) as per Bshary (2001; electronic supplementary material, table S1).
Differences between cleanerfish species in cheating or punishment could stem from differences in the composition of their clientele. A multivariate community composition analysis revealed that the clienteles of L. dimidiatus and L. bicolor were indeed different (see the electronic supplementary material). Therefore to minimize the potential effect of variable clientele composition, we considered only the 32 species of clients inspected by both species of cleanerfish in subsequent analyses (see electronic supplementary material, table S1, for client species lists). These shared clients species represented 83 per cent of all clients inspected by L. dimidiatus and 99 per cent of L. bicolor clients.
We used an ANOVA to examine the effects of cleanerfish age (fixed factor: juvenile, sub-adult, adult), as a proxy for home range size, and cleanerfish species (random factor: L. dimidiatus and L. bicolor) on the prevalence of cheating by cleaners and retaliatory chases by clients. The prevalence of punishment by clients was square root-transformed to meet the assumption of normality. As described above, simple-effects analyses (MANOVA) were used to examine the interaction terms. This analysis was then repeated for resident and non-resident clients separately.
3. Results
(a). Cleanerfish body and home range sizes
A total of 57 bluestreak cleaner wrasses and 43 bicolor cleaner wrasses were observed.
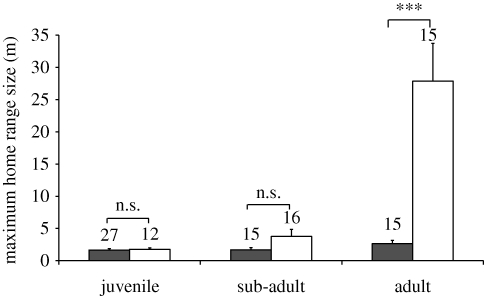
Differences in home range size across age groups varied significantly between species (two-way ANOVA: species: F1,94 = 2.52, p = 0.25; age: F2,94 = 2.30, p = 0.30; species * age: F2,94 = 12.05, p < 0.001; figure 1). Adult L. bicolor had larger home ranges than adult L. dimidiatus (MANOVA: F1,98 = 31.78, p < 0.001), whereas the home ranges of sub-adults and juveniles did not differ between the two cleanerfish species (MANOVA, sub-adults: F1,98 = 1.47, p = 0.23; juveniles: F1,98 = 3.37, p = 0.70). Within L. bicolor, adults had larger home ranges than sub-adults and juveniles (Bonferroni post hoc tests: p < 0.001), but home range size did not differ between L. bicolor juveniles and sub-adults (Bonferroni post hoc test: p = 1.00), nor with age in L. dimidiatus (ANOVA: F2,54 = 2.49, p = 0.09).
Figure 1.
Home range size (in m moved in 30 min) for cleanerfish of different species (L. dimidiatus: filled bars; L. bicolor: open bars) and age classes. Sample sizes are indicated above each bar. ***p < 0.001 and n.s. = p > 0.05 from MANOVA simple-effects analyses.
(b). Cleanerfish cheating and client retaliation
Overall, L. dimidiatus inspected more than twice as many clients per 30 min as L. bicolor (mean ± s.e.: L. dimidiatus = 38.2 ± 2.2; L. bicolor = 15.3 ± 1.3; ANOVA, F1,94 = 43.01, p < 0.001). Labroides dimidiatus inspected 68 reef fish species. Labroides bicolor inspected 35 species, 32 of which were in common with L. dimidiatus (electronic supplementary material, table S1). When only these shared clients are considered, L. dimidiatus still inspected clients at twice the rate of L. bicolor (mean ± s.e.: L. dimidiatus = 30.4 ± 2.3; L. bicolor = 15.2 ± 1.3; ANOVA, F1,97 = 27.0, p < 0.001). All subsequent analyses pertain only to shared clients.
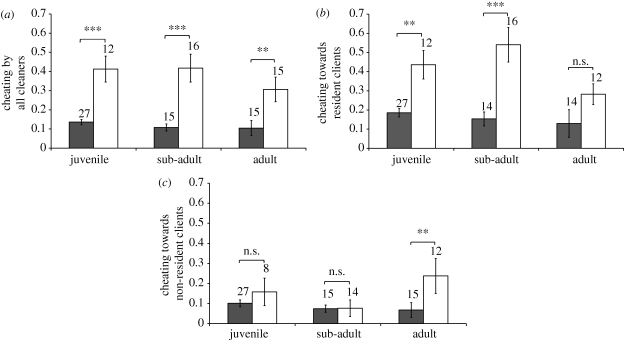
The prevalence of cheating by cleanerfish varied between the two cleaner species, but not among age classes (table 1 and figure 2a). Labroides bicolor cheated a greater proportion of clients than L. dimidiatus. When resident and non-resident clients are examined separately, a different pattern emerges. Labroides bicolor cheated on a significantly higher proportion of its resident clients than did L. dimidiatus (table 1 and figure 2b), but this interspecific difference in cheating was not observed for non-resident clients (table 1 and figure 2c). For both resident and non-resident clients, the prevalence of cheating by cleaners did not vary across ages, and there was no interaction between cleaner age and cleaner species (table 1).
Table 1.
Results of analyses of variance of prevalence of cheating by cleanerfish (i.e. proportion of visiting clients that jolted in response to cleanerfish inspection) on all clients combined, resident clients only and non-resident clients only. One fixed factor, cleaner age (juvenile, sub-adult, adult), and one random factor, cleanerfish species (L. dimidiatus and L. bicolor) and their interaction were estimated. d.f., degrees of freedom; F, test statistic; p, probability; significant values are in bold.
| prevalence of cheating towards all clients combined |
prevalence of cheating towards resident clients only |
prevalence of cheating towards non-resident clients only |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| cleaner species | 1,94 | 69.17 | 0.01 | 1,89 | 15.72 | 0.05 | 1,85 | 2.31 | 0.27 |
| cleaner age | 2,94 | 1.79 | 0.36 | 2,89 | 1.51 | 0.39 | 2,85 | 0.87 | 0.54 |
| cleaner species * cleaner age | 2,94 | 0.67 | 0.51 | 2,89 | 1.90 | 0.16 | 2,85 | 2.00 | 0.14 |
Figure 2.
Prevalence of cheating (measured as the proportion of inspected clients that jolted) to (a) all clients combined, (b) clients with access to a single cleanerfish (residents) and (c) clients with potential access to more than one cleanerfish (non-residents) for cleanerfish of different species (L. dimidiatus: filled bars; L. bicolor: open bars) and age classes. Sample sizes are indicated above each bar. ***p < 0.001, **p < 0.01 and n.s. = p > 0.05 from MANOVA simple-effects analyses.
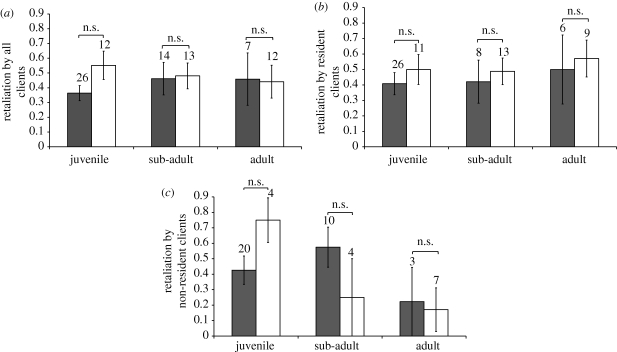
Overall, there were no differences between L. bicolor and L. dimidiatus in the proportion of jolting clients retaliating (table 2 and figure 3a). Cleanerfish age and the interaction between cleaner species and age did not affect the prevalence of retaliatory chases by all clients combined (table 2). However, a significantly higher proportion of cheated residents chased L. bicolor than L. dimidiatus after a jolt (table 2 and figure 3b). This interspecific difference was absent for non-resident clients (table 2 and figure 3c), and there was no overall effect of cleaner age on retaliation by either type of client (table 2).
Table 2.
Results of analyses of variance of prevalence of retaliatory punishment (i.e. square root-transformed proportion of jolting clients that chased the cheating cleaner) by all clients combined, resident clients only and non-resident clients only. One fixed factor, cleaner age (juvenile, sub-adult, adult), and one random factor, cleanerfish species (L. dimidiatus and L. bicolor), and their interaction were estimated. d.f., degrees of freedom; F, test statistic; p, probability; significant values are in bold.
| prevalence of retaliation by all cheated clients |
prevalence of retaliation by cheated resident clients |
prevalence of retaliation by non-resident clients |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| cleaner species | 1,79 | 7.32 | 0.09 | 1,67 | 18.13 | 0.01 | 1,42 | 0.03 | 0.88 |
| cleaner age | 2,79 | 2.19 | 0.31 | 2,67 | 0.08 | 0.93 | 2,42 | 1.09 | 0.48 |
| cleaner species * cleaner age | 2,79 | 0.22 | 0.80 | 2,67 | 0.09 | 0.91 | 2,42 | 2.69 | 0.08 |
Figure 3.
Prevalence of punishment (measured as the proportion of jolting clients that chased the cheating cleanerfish) by (a) all cheated clients combined (b) cheated clients with access to a single cleanerfish (residents, filled bars) and (c) cheated clients with potential access to more than one cleanerfish (non-residents, open bars). Bar heights and errors represent back-transformed mean ± 1 s.e. n.s. = p > 0.05 from MANOVA simple-effects analyses.
4. Discussion
Cleanerfish are often dishonest. Our study confirms that cleaner wrasses often inflict jolt-inducing bites on fish clients, and such client reactions have previously been shown to reflect cheating by various species of cleaners (Bshary & Grutter 2002a; Soares et al. 2008). We had predicted that cleaners that roam widely, such as L. bicolor, would cheat more than site-attached cleaners such as L. dimidiatus. Conversely, we had expected that the clients of roaming cleanerfish would expend little effort in punishing cheating cleaners because the likelihood of repeated encounters should be low. Interspecific comparisons of cleaner wrasses supported our prediction about cheating, but not about punishment. However, intraspecific patterns of cleaner cheating suggest the influence of factors other than home range size differences between cleaners.
Our predictions were premised on the assumption of differences in home range sizes between cleanerfish of different species and ages, which would reflect the likelihood of repeated interactions between individual cleaners and clients. Overall, home range sizes were larger for L. bicolor than for L. dimidiatus (figure 1), which is consistent with reports of station-based cleaning in L. dimidiatus and wider ranging roaming in L. bicolor (Randall 2005). However, home ranges changed differently with ontogeny in the two cleanerfish species. Home range size remained relatively constant, at approximately 2 m in linear distance, across all ages of L. dimidiatus. By contrast, it increased with age in L. bicolor, such that the typical distance travelled by adult L. bicolor during the course of a 30 min observation was more than 10 times as large as that travelled by adult L. dimidiatus. Given that L. bicolor inspected only half as many clients as did L. dimidiatus, the likelihood of repeat encounters with the same client may be generally lower for the former. This will be particularly true for adult L. bicolor since their cleaning encounters occurred over a larger reef area. Our predictions of increased cheating by cleanerfish and lower punishment by clients should therefore be most clearly demonstrated in the behaviour of adult L. bicolor and their clients.
As predicted, L. bicolor cheated more than L. dimidiatus (figure 2a). Overall, approximately 12 per cent of clients inspected by L. dimidiatus exhibited body jolts, indicative of dishonest cleaning (Bshary & Grutter 2002a; Soares et al. 2008), while approximately 38 per cent of clients inspected by L. bicolor jolted. This interspecific difference was driven largely by the particularly dishonest behaviour of L. bicolor towards its resident clients. Clients with small home ranges may generally be vulnerable to cheating because they lack choice options when seeking to interact with a cleaner. Asymmetries in partner choice increase the likelihood that one partner—the one with more choice, which, in this case, is the cleaner—will cheat (Noë & Hammerstein 1994; Bshary & Grutter 2002a). However, it is not clear that the asymmetry in partner choice is greater between clients and L. bicolor than with L. dimidiatus because of cleaner home range differences since there was no association between the prevalence of cheating and cleanerfish age (table 1). More specifically, despite having the largest home ranges, adult L. bicolor did not cheat more than younger conspecifics. It is possible that cheating is in fact related to cleanerfish roaming behaviour, but we underestimated home range size for younger L. bicolor and they actually roam as extensively as adult conspecifics do. We feel that this is unlikely given that observation methods were standardized across all cleanerfish. Alternatively, adult L. bicolor do cheat more than adult L. dimidiatus because they roam but the high prevalence of cheating by younger L. bicolor has an alternative explanation. The unexpected high level of cheating by young L. bicolor could be caused, for example, by the composition of their clientele. In fact, the clientele of juvenile L. bicolor comprised significantly more resident than non-resident clients, a difference that was not observed in other age groups of either cleanerfish species (electronic supplementary material, table S2 and figure S1).
Contrary to our expectation, the prevalence of retaliation was similar between the cheated clients of L. bicolor and L. dimidiatus, and across all cleaner age classes (figure 3a). However, a greater proportion of resident clients that received jolt-inducing bites from L. bicolor chased their cleaners compared with those that interacted negatively with L. dimidiatus (figure 3b and table 2). The reason for this difference in propensity to chase cheating cleaners is unclear. One possibility is that the lower tolerance of cheating by clients of L. bicolor reflects a higher cost of being cheated on by L. bicolor than by L. dimidiatus, perhaps because it inflicts more painful or damaging bites. At any rate, a clear link between the prevalence of retaliation by cheated clients and the extent of cleaner roaming is again absent since the prevalence of retaliatory chasing was not lowest against adult L. bicolor, i.e. the cleaners with the largest home ranges and hence the lowest likelihood of repeated interactions with individual clients.
Our results give rise to a puzzling question. Why do fish clients continue to interact with L. bicolor, given that a generally more honest cleanerfish species (i.e. L. dimidiatus) is present on the reef? The answer may lie in the difficulty faced by many fish, particularly those with small home ranges or territories, in gaining access to a cleanerfish. While those residing near cleaning stations operated by L. dimidiatus have frequent and inexpensive access to an honest cleaner, fish living further away do not (see Cheney & Côté (2001) for a Caribbean example). For these fish, the cost of being cheated by a more dishonest cleaner like L. bicolor may still be lower than the costs of travelling (e.g. time, aggression by other territorial fish, intrusions onto temporarily abandoned territories) to visit a more honest cleaner like L. dimidiatus. Thus, to be cleaned by a cleaner that sometimes cheats may be a better option than not to be cleaned at all.
Acknowledgements
We would like to thank David Lecchini, Christophe Brié, Tropical Fish Tahiti and Service de la Perliculture of French Polynesia for kindly accommodating this project and us. This research was financially supported by grants from Agence National pour la Recherche (ANR-06-JCJC-0012-01) and CRISP programme (Coral Reef Initiative in the South Pacific—C2A1) and by a Simon Fraser University President's Research Grant to IMC.
References
- Axelrod R., Hamilton W. D.1981The evolution of cooperation. Science 211, 1390–1396 (doi:10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- Bergmuller R., Johnstone R. A., Russell A. F., Bshary R.2007Integrating cooperative breeding into theoretical concepts of cooperation. Behav. Process. 76, 61–72 (doi:10.1016/j.beproc.2007.07.001) [DOI] [PubMed] [Google Scholar]
- Bronstein J. L.1991The nonpollinating wasp fauna of Ficus pertusa—exploitation of a mutualism. Oikos 61, 175–186 (doi:10.2307/3545335) [Google Scholar]
- Bronstein J. L.2001The exploitation of mutualisms. Ecol. Lett. 4, 277–287 (doi:10.1046/j.1461-0248.2001.00218.x) [Google Scholar]
- Bshary R.2001The cleaner fish market. In Economics in nature (eds Noë R., van Hoof J. A. R. A. M., Hammerstein P.), pp. 146–172 Cambridge, UK: Cambridge University Press [Google Scholar]
- Bshary R.2002aBiting cleaner fish use altruism to deceive image-scoring client reef fish. Proc. R. Soc. Lond. B 269, 2087–2093 (doi:10.1098/rspb.2002.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R.2002bBuilding up relationships in asymmetric co-operation games between the cleaner wrasse Labroides dimidiatus and client reef fish. Behav. Ecol. Sociobiol. 52, 365–371 [Google Scholar]
- Bshary R., Grutter A. S.2002aAsymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 63, 547–555 (doi:10.1006/anbe.2001.1937) [Google Scholar]
- Bshary R., Grutter A. S.2002bExperimental evidence that partner choice is a driving force in the payoff distribution among cooperators or mutualists: the cleaner fish case. Ecol. Lett. 5, 130–136 (doi:10.1046/j.1461-0248.2002.00295.x) [Google Scholar]
- Bshary R., Grutter A. S.2005Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol. Lett. 1, 396–399 (doi:10.1098/rsbl.2005.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R., Schäffer D.2002Choosy reef fish select cleaner fish that provide high-quality service. Anim. Behav. 63, 557–564 (doi:10.1006/anbe.2001.1923) [Google Scholar]
- Bshary R., Würth M.2001Cleaner fish Labroides dimidiatus manipulate client reef fish by providing tactile stimulation. Proc. R. Soc. Lond. B 268, 1495–1501 (doi:10.1098/rspb.2001.1495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caullery M.1952Parasitism and symbiosis. London, UK: Sidgwick and Jackson [Google Scholar]
- Cheney K. L., Côté I. M.2001Are Caribbean cleaning symbioses mutualistic? Costs and benefits of visiting cleaning stations to longfish damselfish. Anim. Behav. 62, 927–933 (doi:10.1006/anbe.2001.1832) [Google Scholar]
- Clutton-Brock T. H., Parker G. A.1995Punishment in animal societies. Nature 373, 209–216 (doi:10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- Connor R. C.1995Altruism among non-relatives—alternatives to the prisoners dilemma. Trends Ecol. Evol. 10, 84–86 (doi:10.1016/S0169-5347(00)88988-0) [DOI] [PubMed] [Google Scholar]
- Côté I. M.2000Evolution and ecology of cleaning symbioses in the sea. Oceanogr. Mar. Biol. Annu. Rev. 38, 311–355 [Google Scholar]
- Douglas A. E.2008Conflict, cheats and the persistence of symbioses. New Phytol. 177, 849–858 (doi:10.1111/j.1469-8137.2007.02326.x) [DOI] [PubMed] [Google Scholar]
- Dugatkin L. A., Wilson D. S.1991Rover—a strategy for exploiting cooperators in a patchy environment. Am. Nat. 138, 687–701 [Google Scholar]
- Enquist M., Leimar O.1993The evolution of cooperation in mobile organisms. Anim. Behav. 45, 747–757 (doi:10.1006/anbe.1993.1089) [Google Scholar]
- Field A.2005Discovering statistics using SPSS. Oxford, UK: The Alden Press [Google Scholar]
- Fitter A. H.2006What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol. 172, 3–6 (doi:10.1111/j.1469-8137.2006.01861.x) [DOI] [PubMed] [Google Scholar]
- Grutter A. S.1997Spatiotemporal variation and feeding selectivity in the diet of the cleaner fish Labroides dimidiatus. Copeia, 1997, 346–355 (doi:10.2307/1447754) [Google Scholar]
- Grutter A. S., Bshary R.2003Cleaner wrasse prefer client mucus: support for partner control mechanisms in cleaning interactions. Proc. R. Soc. Lond. B 270, S242–S244 (doi:10.1098/rsbl.2003.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter A. S., Bshary R.2004Cleaner fish, Labroides dimidiatus, diet preferences for different types of mucus and parasitic gnathiid isopods. Anim. Behav. 68, 583–588 (doi:10.1016/j.anbehav.2003.11.014) [Google Scholar]
- Kiers E. T., Rousseau R. A., West S. A., Denison R. F.2003Host sanctions and the legume-rhizobium mutualism. Nature 425, 78–81 (doi:10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- McDade L. A., Kinsman S.1980The impact of floral parasitism in two Neotropical hummingbird-pollinated plant species. Evolution 34, 944–958 (doi:10.2307/2408000) [DOI] [PubMed] [Google Scholar]
- Mooring M. S., Mundy P. J.1996Interactions between impala and oxpeckers at Matobo National Park, Zimbabwe. Afr. J. Ecol. 34, 54–65 (doi:10.1111/j.1365-2028.1996.tb00594.x) [Google Scholar]
- Noë R., Hammerstein P.1994Biological markets—supply-and-demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11 (doi:10.1007/BF00167053) [Google Scholar]
- Pellmyr O.2002Pollination by animals. In Plant–animal interactions (eds Herrera C. M., Pellmyr O.), pp. 157–184 Oxford, UK: Blackwell Science [Google Scholar]
- Pellmyr O., Huth C. J.1994Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372, 257–260 (doi:10.1038/372257a0) [Google Scholar]
- Potts G. W.1973Ethology of Labroides dimidiatus (Cuv. and Val.) (Labridae, Pisces) on Aldabra. Anim. Behav. 21, 250–291 (doi:10.1016/S0003-3472(73)80068-5) [Google Scholar]
- Randall J. E.2005Reef and shore fishes of the South Pacific: New Caledonia to Tahiti and the Pitcairn Islands. Honolulu, Hawai'i: University of Hawai'i Press [Google Scholar]
- Semple S., McComb K.1996Behavioural deception. Trends Ecol. Evol. 11, 434–437 (doi:10.1016/0169-5347(96)20068-0) [DOI] [PubMed] [Google Scholar]
- Soares M. C., Bshary R., Cardoso S. C., Cote I. M.2008The meaning of jolts by fish clients of cleaning gobies. Ethology 114, 209–214 (doi:10.1111/j.1439-0310.2007.01471.x) [Google Scholar]
- Trivers R. L.1971Evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 [Google Scholar]
- Yu D. W., Pierce N. E.1998A castration parasite of an ant–plant mutualism. Proc. R. Soc. Lond. B 265, 375–382 (doi:10.1098/rspb.1998.0305) [Google Scholar]