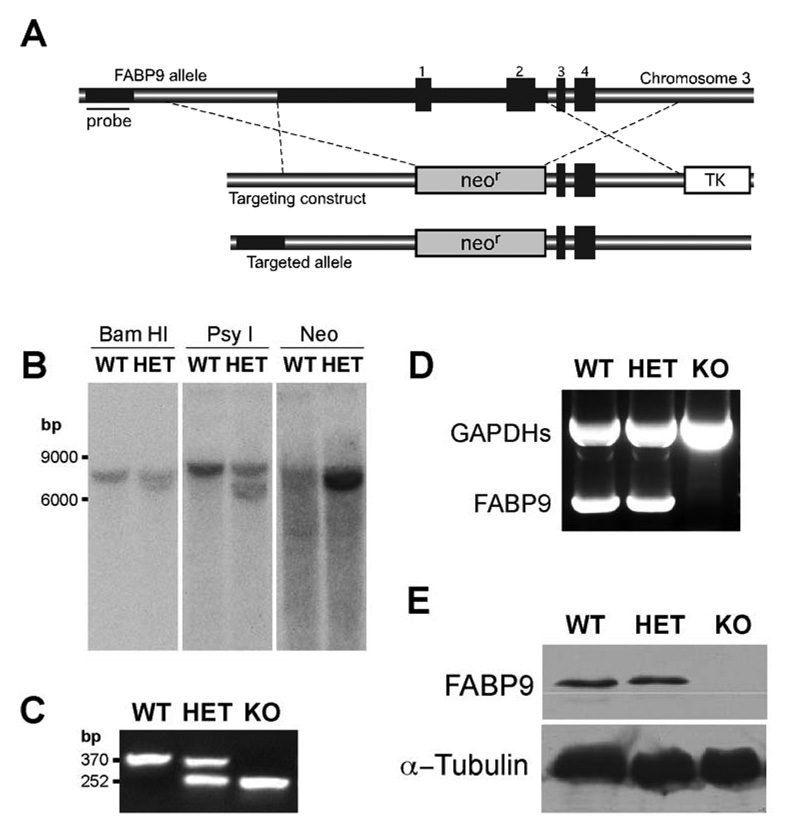
Fig. 3. Targeted disruption of the murine FABP9 gene.
(A) Schematic showing the FABP9 locus, the targeting construct and homologous recombination. The FABP9 gene consists of 4 exons (indicated 1–4). The targeting construct was designed with upstream and downstream regions of homology on either side of a neomycin resistance cassette (neor) replacing exons 1 and 2. In this construct, the neor served for positive selection and a thymidine kinase cassette (TK) for negative selection. The correctly targeted FABP9 allele shows integration of the neor cassette in the genomic locus. The sequence upstream of the recombination site used for screening correctly targeted clones is indicated (Probe). (B) Screening for the targeted FABP9 locus using southern blots. Genomic fragments generated by digestion using BamHI and PsyI were probed for a shift in size of the targeted fragment. The correctly targeted clone was subsequently verified using a neo probe for a single and specific recombination event. (C) Gel showing genotyping PCR for verifying the targeted FABP9 locus. The WT allele produced a 370 bp band and the KO allele produced a 252 bp band. (D) Gel showing RT-PCR products for FABP9 mRNA expression in WT, HET and KO testis. FABP9 mRNA was not expressed in KO mice. A germ cell specific glyceraldehyde 3-phosphate dehydrogenase (GAPDHs) was used as a reaction control. (E) FABP9 immunoblot showing protein expression in WT, HET and KO testis extracts. There was no FABP9 expressed in KO mice. HET mice where one allele of the FABP9 gene was disrupted produced a comparable level of expression to WT mice.