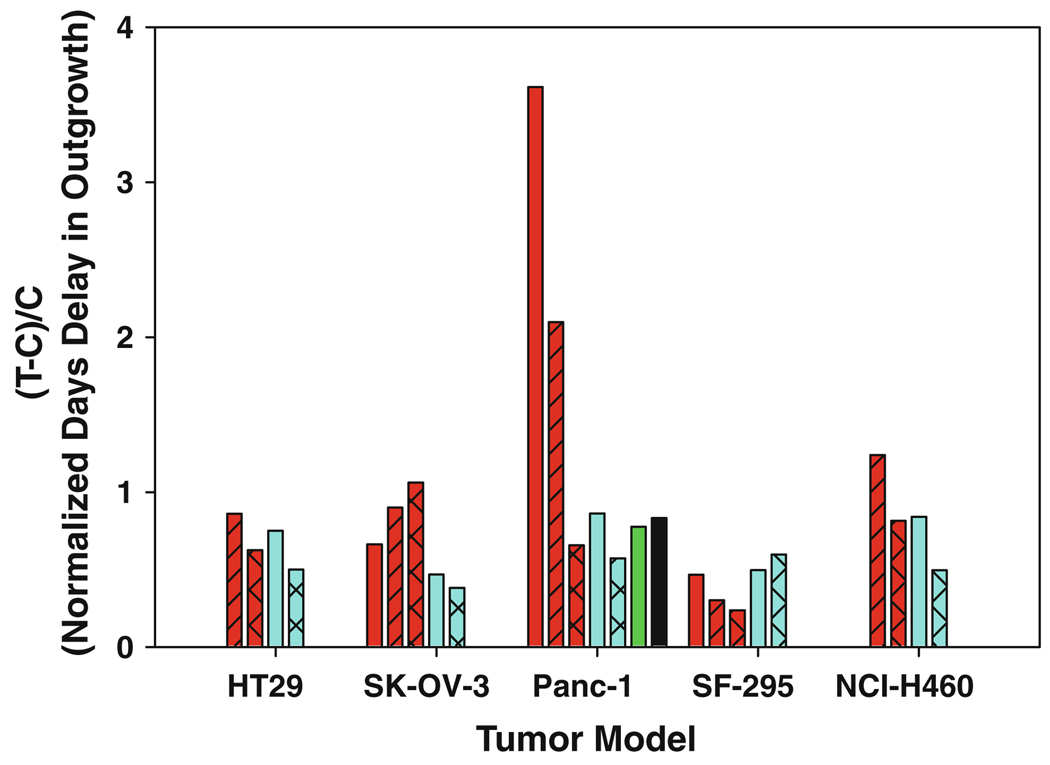
Fig. 3.
Antitumor activity of BACPTDP in human tumor xenografts. Murine xenograft models for human colon (HT29), ovarian (SK-OV-3), pancreatic (Panc-1), glioma (SF-295) and non-small-cell lung (NCI-H460) cancers were treated with BACPTDP (red bars) at 4.5 (cross-hatched), 6.7 (hatched), or 8.0 mg/kg (no hatch) on a 5/2/5 schedule and run head-to-head against irinotecan (CPT-11; blue bars) at 40 (hatched) and 60 (no hatch) mg/kg on a Q4D×3 schedule. Data for BACPTDP at 8 mg/kg are not included for the NCI-H460 xenograft due to increased toxicity in this model. Results are expressed as the overall delay in growth of the median tumor (T–C; T treated group, C control group), normalized to account for diferent tumor doubling times (two for SK-OV-3 and Panc-1; three for HT-29 and NCI-H460; four for SF-295). For comparison, published values are included for gemcitabine (black bar) and rubitecan (green bar) at their maximum tolerated doses of 60 mg/kg and at 2.5 mg/kg, respectively, in the Panc-1 model