Table 3.
N-Sulfonyl Triazole Synthesis: Scope with Respect to Sulfonyl Azidea

| entry | R1 | alkyne | product | time (h) | yield (%)b |
|---|---|---|---|---|---|
| 1c | 4-BrC6H4 |

|
13 | 3 | 86 |
| 2 | 4-BrC6H4 |

|
23 | 4.5 | 78 |
| 3d | 4-H3COC6H4 |
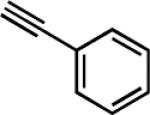
|
14 | 6.5 | 94 |
| 4 | 4-H3COC6H4 |

|
24 | 7 | 79 |
| 5 | Bn |

|
25 | 3.5 | 87 |
| 6e | Me |
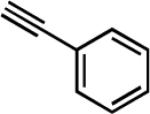
|
26 | 12 | 88 |
| 7e | i-Pr |
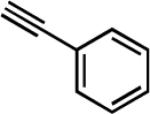
|
27 | 12 | 75 |
| 8 | n-C8H17 |

|
28 | 3.5 | 92 |
| 9f |
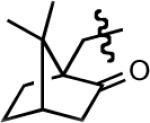
|
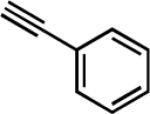
|
29 | 8.5 | 90 |
| 10g |

|
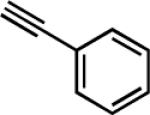
|
30 | 3 | 82 |
| 11g |
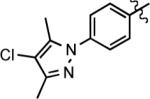
|

|
31 | 3 | 83 |
1.0 mmol scale unless otherwise noted; CuTC (10 mol %), solvent (0.2 M), alkyne (1 equiv), sulfonyl azide (1 equiv), rt.
Isolated yield.
5.0 mmol scale.
1.1 equiv alkyne.
1.3 equiv alkyne.
2.4 equiv alkyne.
1.2 equiv alkyne.
