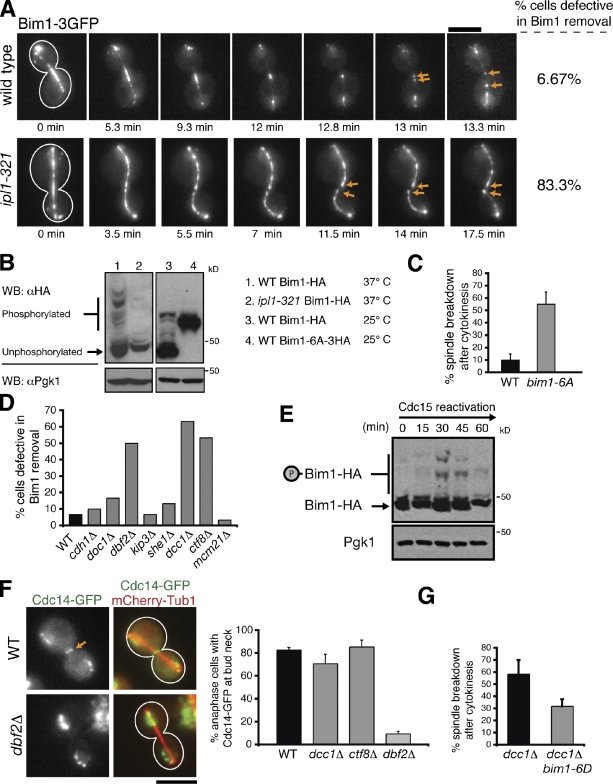
Figure 5.
The MEN and the A-RFC complex regulate Ipl1-mediated removal of Bim1 from the spindle midzone. (A) Time-lapse fluorescence images of Bim1-3GFP localization in wild-type and ipl1-321 cells incubated at 37°C. Arrows indicate Bim1-3GFP decoration of the plus ends of spindle halves after disassembly onset. (B) Protein extracts isolated from asynchronous populations of the indicated strains were separated by SDS-PAGE supplemented with 30 µM Phos-tag acrylamide. The tagged phosphomutant version of Bim1 (Bim1-6A-3HA) contained a larger C-terminal epitope and a longer linker than Bim1-HA; thus, Bim1-6A-3HA migrates slower than Bim1-HA. The presence of a single Bim1-6A-3HA band indicates the absence of phosphorylation. Pgk1 was used as a loading control. WB, Western blotting. (C) Frequency in which spindles disassembled after initiation of cytokinetic ring contraction in the indicated cells. (D) Bim1-3GFP localization just before spindle disassembly in the indicated strains (n = 30 for each). Only dbf2Δ, ctf8Δ, and dcc1Δ mutants displayed pronounced defects in Bim1 removal. (E) cdc15-2 cells expressing Bim1-HA were synchronized in late anaphase after a 3-h incubation at 37°C. These cells were then released by lowering the temperature to 23°C, and cells were harvested every 15 min. Protein extracts were separated by SDS-PAGE supplemented with 30 µM Phos-tag acrylamide. Pgk1 was used as a loading control. (F) In wild-type (WT), dcc1Δ, and ctf8Δ cells, Cdc14-GFP was exported from the nucleus and localized to the bud neck (arrow) during late anaphase. However, Cdc14-GFP export was defective in dbf2Δ cells (n = 75). (G) Expression of bim1-6D alleviates the spindle disassembly defects in dcc1Δ cells. (C and G) n = 3 experiments, with 20 events per experiment. Error bars indicate mean ± SD. Bars, 5 µm.