Figure 3. Mapping the local HDX changes on a ribbon model of the Arp3-Arp2 dimer.
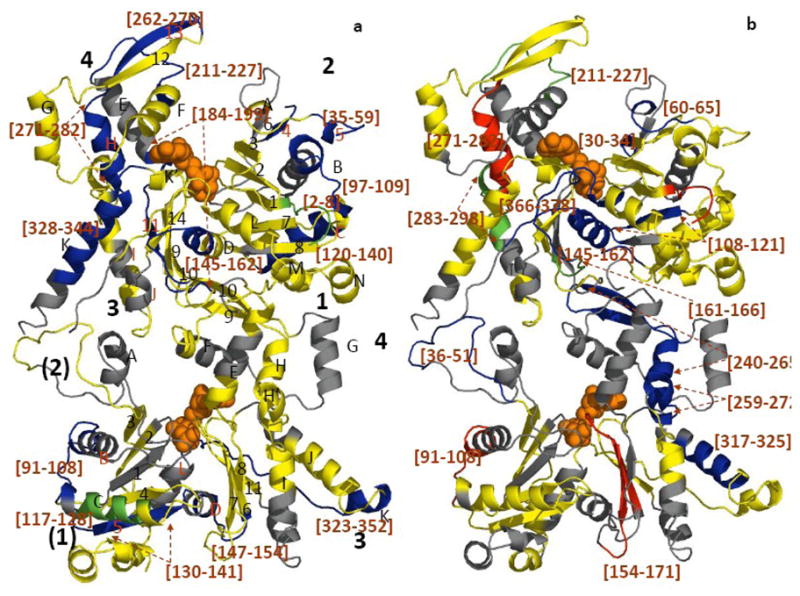
a) compares deuterium exchange into individual peptic peptides with and without ATP; b) compares deuterium exchange into individual peptides of ATP-Arp2/3 complex with and without WASp-VCA.) Differences in deuterium incorporation were determined by the mass difference in individual peptides between a) the apo- and the ATP-bound complex and b) the ATP- and ATP/WASp-bound complex. Subdomains of Arp2 and Arp3 are labeled 1–4. Arp3 (top) is based on the crystal structure (1TYQ) and the Arp2 (bottom) is from the composite structure of 1TYQ with modeled subdomains 1 and 2. α-helices are labeled with capital letters and β-sheets are labeled with numbers in a). Segments of the polypeptide chains cleaved by pepsin are labeled by degree of protection: red >+0.5 Da; yellow, −0.5 Da to 0.5 Da; green, −0.5 to −1 Da; blue <−1 Da; and grey, missing peptides. Peptides with significant changes in deuterium incorporation are labeled (brackets).
