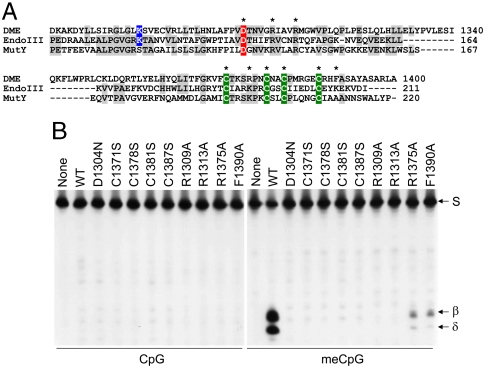
Fig. 1.
Importance of the [4Fe-4S] cluster of DME for 5mC excision. (A) A HhH-GPD/Fe-S cluster of DME is structurally similar to those of EndoIII and MutY in E. coli. Besides a conserved HhH motif, four cysteine residues (green) that constitute the [4Fe-4S] cluster and some other functionally important residues are highly conserved among these three proteins. Catalytically important aspartic acid (canonical in this family) and lysine (specific to bifunctional enzymes with glycosylase/AP-lyase activities) residues are colored in red and blue, respectively. The amino acid residues subjected to site-directed mutagenesis are indicated with asterisks. (B) In vitro 5mC excision activity of [4Fe-4S] cluster mutant DME proteins. Active MBP-DMEΔN677 (WT) and proteins with indicated amino acid substitutions were reacted with unmethylated (Left) or methylated (Right) oligonucleotide substrates. Oligonucleotide substrate (S) and β- and δ-elimination products are indicated to the right of the panel.