Abstract
Downscaling from the predictions of general climate models is critical to current strategies for mitigating species loss caused by climate change. A key impediment to this downscaling is that we lack a fully developed understanding of how variation in physical, biological, or land-use characteristics mediates the effects of climate change on ecological communities within regions. We analyzed change in understory herb communities over a 60-y period (1949/1951–2007/2009) in a complex montane landscape (the Siskiyou Mountains, Oregon) where mean temperatures have increased 2 °C since 1948, similar to projections for other terrestrial communities. Our 185 sites included primary and secondary-growth lower montane forests (500–1.200 m above sea level) and primary upper montane to subalpine forests (1,500–2,100 m above sea level). In lower montane forests, regardless of land-use history, we found multiple herb-community changes consistent with an effectively drier climate, including lower mean specific leaf area, lower relative cover by species of northern biogeographic affinity, and greater compositional resemblance to communities in southerly topographic positions. At higher elevations we found qualitatively different and more modest changes, including increases in herbs of northern biogeographic affinity and in forest canopy cover. Our results provide community-level validation of predicted nonlinearities in climate change effects.
Keywords: climate change, elevation, land use, plant community, topography
Upward and poleward shifts of species and vegetation zones are expected under climatic warming, and considerable evidence has been found in support of these broad predictions (1–5). However, large differences among communities in the magnitude, rate, and direction of responses to climatic warming are also expected, based on factors such as topography and substrate, land-use history, and community-level variation in species functional traits (e.g., 6, 7–10). Anticipating ecological contingency in responses to climate change is especially critical for managers of natural resources, who are well aware of the potential for major nonlinearities (“surprises”) in community change and of the particular difficulty of making predictions for physically and biotically complex landscapes (e.g., 11–13). Our ability to test and refine predictions about contingent changes over time is limited by the scarcity of both appropriate data sets and metrics for comparisons across ecological communities.
One of the earliest and best-known expectations about contingency is that climate change effects should be most pronounced at high elevations where plant growth is most strongly limited by temperature (14), specifically by the length of the snow-free growing season. This expectation is based on studies in the alpine and nival zones of the European Alps and elsewhere, where warming temperatures have been observed to lead to increases in plant productivity and species richness, although with losses of high-elevation specialist species, presumably as the result of competition (e.g., 9, 14–16). However, growing evidence also shows severe effects of climatic warming in warm and water-limited biomes, such as the western United States at low to moderate elevations, where enhanced drought stress has led to widespread vegetation die-off (e.g., 2, 17, 18). In fact, physiologically based models predict that a given amount of growing-season warming should reduce plant growth at water-limited low elevations while enhancing it at temperature-limited higher elevations (e.g., 12, 19), a straightforward prediction that accords with data on tree growth rates (11), remotely sensed productivity indices (20), and shifts in flowering phenology (21). In turn, such an elevational contrast in key limiting factors is a major reason to expect that climatic warming will produce nonlinear responses rather than the smooth upward shifts predicted by simple models; however, this expectation has seldom been tested with data on change in actual communities over time.
Human land-use history is another important factor influencing the sensitivity of ecological communities to climate change. Although there is a general expectation that many human activities will interact with climate change to worsen its impacts on biodiversity (e.g., 22–24), it is possible to make more nuanced predictions based on whether a specific human impact increases, decreases, or acts independently of the role of climatic limiting factors. Just as in the case of elevational contrasts, there have been few opportunities to refine such predictions by comparing change over time in adjacent communities differing in key aspects of land-use history.
Here we couple historical and contemporary data to compare 60 y of change in adjacent communities differing in elevation and land use. In 2007–2009 we resampled, as closely as possible, 185 sites in the Siskiyou Mountains (Oregon) that first were studied by ecologist Robert H. Whittaker in 1949–1951 with the goal of assessing community variation along steep environmental gradients (25). The region is a hotspot of botanical diversity with global significance because of the high endemism associated with its geologic and topographic complexity (25–27). Mean annual temperatures and mean summer temperatures have increased ≈2 °C since 1948, and snow-water equivalent (the product of snow depth and snow relative density) has declined, although mean and seasonal precipitation have not changed significantly (28–30). Clear-cut logging was widespread in the 1960s through the 1980s at low to moderate elevations, leaving a mosaic of secondary forests composed largely of the same species but in altered relative abundances and age classes (31). Our sites include primary (never logged) and secondary-growth (logged in the 1960s through the 1980s) lower montane forests (500–1,200 m above sea level), which do not have a winter snowpack, and primary upper montane to subalpine forests (1,500–2,100 m above sea level), which receive a regular yearly snowpack.
We used three metrics of community change that aggregate across species and thus are especially suitable when beta (among-site) diversity is high and no individual species are either widespread or abundant: (i) the abundance-weighted mean value of specific leaf area (SLA), or leaf area divided by dry mass, the plant morphological trait that varies most consistently among species in relation to environmental moisture balance (species in moist or shady environments have higher SLA than species in arid or sunny environments) (e.g., 32–34); (ii) relative cover by herbs of north temperate (as opposed to semiarid, desert, or unknown) biogeographic affinity, which belong to evolutionary lineages characteristic of cool and moist conditions (35–37); and (iii) community position on an ordination axis paralleling the spatial shift from cool north slopes to warm south slopes (along this axis, daily near-surface temperatures increase by several degrees, and vegetation changes strikingly) (25, 28, 36).
We made the following predictions about ecologically contingent change in herb-community composition between 1949–1951 and 2007–2009. First, we predicted changes in lower montane forests consistent with an effectively drier climate: i.e., lower mean SLA, lower relative cover by northern species, and a shift toward greater resemblance to communities on southerly slopes. Second, we predicted that changes in upper montane to subalpine forests would differ qualitatively from those in lower montane forests; in particular, compositional shifts should not be consistent with drought stress. Fast-growing, highly competitive species might be expected to increase at the expense of less competitive species (although we had limited means to assay this expectation). Third, we predicted that changes caused by human land-use history would be qualitatively distinct from those caused by climatic warming. Specifically, we expected that herb communities in both primary and secondary-growth lower montane forests would change consistently with a drier climate, because a history of logging followed by secondary forest regeneration should not alter the role of moisture as a key limiting factor. Land-use history may have other significant impacts, of course; we tested for differences in cover by late-seral indicator herb species, i.e., those known to regenerate slowly following canopy-removing disturbances such as logging or fire.
Our sites were not affected significantly by exotic species, fire, grazing, or (except in known cases) logging. Succession following fire is an important cause of change in the study region, particularly at low elevations (e.g., 31, 38), although, because our study sites were chosen to be in late-successional condition in 1949–1951 (25), it is unclear how much additional succession to expect at these sites. Warming temperatures also could alter forest canopy cover, with indirect effects on herbs (11, 12). To account for any possible role of successional or other forest canopy changes in causing herb-community changes, we analyzed canopy cover in historic and modern aerial photographs, as well as testing for changes in late-seral indicator herbs, which would be expected to increase during succession.
Results
Our analyses are based on the 181 herb species, all perennial, for which either we in 2007–2009 or Whittaker in 1949–1951 found >0% cover in at least one of the 185 plots. Percent of cover was measured as the number of 1 × 1-m subplot corners (out of 100) that each herb species intercepted within a 50 × 20-m plot. All these 181 species were native to the region, because neither we nor Whittaker found any exotic species common enough to register a cover value of >0%. Dominance was low in these communities; median (minimum-maximum) cover values by herb species per plot were 2.7% (1.0–11.0%), 3.3% (1.0–12.5%), and 2.9% (1.0–16.0%) for lower montane primary forest, lower montane secondary forest, and upper montane-subalpine primary forest, respectively. Therefore, rather than using particular indicator species, we used our three metrics based on traits, biogeography, and ordination to examine the aggregate responses of these species.
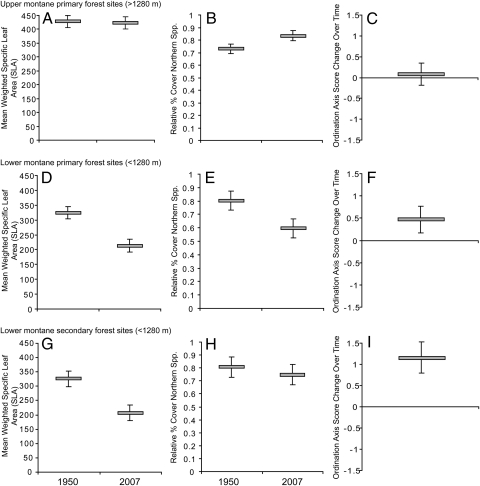
In a multivariate analysis, all three metrics showed significant warming-consistent shifts in community composition. In both primary and secondary lower montane forest, but not in upper montane to subalpine primary forest, we found decreased abundance-weighted mean SLA (Fig. 1). In primary lower montane forests, we also found a significant decline over time in relative cover by species belonging to families or genera of north temperate biogeographic origin. This decrease was nonsignificant in secondary lower montane forests, and the change was in the opposite direction in primary upper montane to subalpine forests (Fig. 1).
Fig. 1.
Plant-community changes related to climatic warming. Means and 95% confidence limits are shown for the weighted abundance of SLA per plot, relative percent of cover by species with northern biogeographic affinities, and the change in ordination axis score values for changes caused by climatic position in the environment. Means are shown for each response variable for upper montane primary forest sites (A–C), lower montane primary forest sites (D–F), and lower montane secondary-growth forest sites (G–I). These three multiple response variables changed significantly over time as a function of community type (MANOVA, Pillai's trace; F = 18.16; df = 6, 256; P < 0.001).
In both primary and secondary lower montane forests, we found warming-consistent changes in community composition in ordination space. Using nonmetric multidimensional scaling (NMS) implemented in PC-Ord (39, 40), we separately ordinated herb communities in lower montane forest (both primary and secondary-growth, because they are compositionally similar) and upper montane and subalpine forest, combining Whittaker's (1949–1951) data and our own (2007–2009) data in each ordination. We rotated the first axis of each ordination to maximize its correlation with the biophysical gradient from cool northerly slopes to warm southerly slopes. The difference in the axis 1 score of each community in Whittaker's (1949–1951) data and our (2007–2009) data were used as a response variable in our multivariate analysis of variance (MANOVA). We found significant shifts toward higher axis 1 scores in the low-elevation forests (Fig. 1), meaning that their herb composition today, compared with Whittaker's time, has become more like that of a warm south-facing slope. Paralleling the results based on SLA, this shift was found in primary and secondary lower montane forest but not in primary upper montane to subalpine forest (Fig. 1).
These compositional changes were driven by small declines in the abundances of large numbers of species, with no species showing substantial increases in abundance (Fig. S1). Species richness declined more in primary and secondary low-elevation forests (which did not differ significantly from each other) than in high-elevation forests (F = 5.44, P = 0.02), whereas declines in total cover did not differ between low and high elevations (F = 0.45, P = 0.51). Although the abundance-weighted community mean SLA declined in lower-elevation forests, the distribution of SLA values unweighted by species abundances did not change (χ2, P > 0.10) (Fig. S2), again pointing to altered relative abundances rather than species gains and losses as the cause of the observed change.
We found no change in the relative cover by herbs considered to be indicators of late-seral forest conditions in either primary lower montane forest (t = 0.65, P = 0.52) (Fig. S3) or upper montane to subalpine forest (t = 0.19, P = 0.85) (Fig. S3), suggesting these forests already were in late-successional condition in 1949–1951 (consistent with Whittaker's description of his site selection; ref. 25). However, the secondary lower montane forests that were logged in the 1960s through the1980s showed the expected lower percent of cover by late-seral indicator herbs in 2007–2009 compared with 1949–1951 (t = 4.93, P < 0.001) (Fig. S3).
In analyses using aerial photographs from 1940 and 2005 (41), mean canopy cover showed no change in primary lower montane forest (t = 0.95; df = 1, 3; P = 0.39) (Fig. S3), a slight and marginally significant decrease in secondary lower montane forest (t = 1.01; df = 1, 23; P = 0.06) (Fig. S3), and a significant increase in the primary upper montane to subalpine forest (t = 8.41; df = 1, 43; P < 0.001) (Fig. S3). These patterns are inconsistent with forest succession as a cause of the observed herb-community changes at low elevations, although they suggest a possible role for warming-induced canopy expansion at high elevations. We did not find significant correlations between forest-cover change and herb-community composition (P > 0.05 for all regressions of change in canopy cover and change in mean SLA, relative cover by northern taxa, or axis 1 ordination scores), nor was forest-cover change correlated with change in total herb cover (P = 0.13, R2 = 0.59 for primary lower montane communities; P = 0.18, R2 = 0.08 for secondary-growth lower montane communities; and P = 0.50, R2 = 0.01 for primary upper montane communities) or species richness (P = 0.12, R2 = 0.61; P = 0.95, R2 < 0.01; and P = 0.23, R2 = 0.04 for these communities, respectively).
Discussion
The herb-community changes we observed in lower montane forests are consistent with the 2 °C increase in mean seasonal and annual temperatures in the study region since 1948 (28–30) as well as with climatic and ecological trends in many other parts of the western United States (e.g., 17, 18, 42). The primary effect of climatic warming in water-limited environments, in the absence of changes in precipitation, is to exacerbate drought stress (5, 17, 18). Accordingly, we found that Siskiyou low-elevation forest herb communities have shifted toward a greater prevalence of species with small, thick leaves (lower SLA) that are better adapted to dry conditions than species with large, thin leaves (32–34). Undisturbed lower montane herb communities at low elevations also shifted toward lower percentage of cover by species of north temperate biogeographic origin, which are characteristic of cool and moist macro- and microenvironments (35–37, 43, 44). Finally, both undisturbed and second-growth lower montane herb communities shifted to resemble more closely communities on warm southerly slopes, as we previously found for herb communities on serpentine soils in the study region (28). Our study adds to growing ecological evidence for drier forest understory conditions in many parts of the world (e.g., 45, 46–49). The three metrics we developed may prove valuable as a way to compare the effects of climate change across multiple ecological communities having few species in common.
Our study compares the community-level effects of climate change along an elevational gradient and documents a strong elevational nonlinearity in these effects. Given the emphasis that has been placed on high-elevation ecosystems in the climate-change literature (e.g., 9, 14–16, 50), it may seem counterintuitive that we found more pronounced and negative changes at lower elevations. However, our results are in agreement with predictions and observations concerning the shift in effects of warming across the transition from water-limited low elevations to snowpack-limited higher elevations (11, 12, 19–21). At the higher elevations in our study, forest canopy cover showed an increase from Whittaker's time to the present. This finding is unexpected on the basis of normal successional change, because the fire cycle at these elevations is extremely slow (51). Instead, it may be the result of the longer snow-free growing season (e.g., 5, 11, 12, 52). In turn, increased shading from a more vigorous overstory at these elevations may have suppressed herb cover and richness (11, 12) as well as contributing to the observed shift toward shade-tolerant herbs of northern biogeographic affinity. Had our study extended into still higher elevations where there is no forest cover, we would expect to find increases in herb cover and richness similar to those observed in Alpine studies (e.g., 15, 16, 50). The observed elevational patterns may continue to change over time, with water limitation potentially becoming important at progressively higher elevations as the snowpack diminishes or disappears completely.
Because human land-use activities have been predicted to interact with climate change in multiple and mostly negative ways (19, 22, 23), the relative consistency in the changes we observed in primary and secondary lower montane forests also may appear surprising. However, these results simply indicate that the impacts of clear-cut logging and postlogging regeneration on forest herb communities, although they may be severe, may tend neither to enhance nor to mitigate the stresses imposed by a warming and drying climate. Rather, the main effect of logging on herbs may be to reduce the abundances of species with modes of persistence, dispersal, and regeneration that are ill-adapted to recovery from heavy disturbance (e.g., 31, 53, 54), and this effect acts largely independently of climatic limiting factors.
We considered but found no evidence for other major causes of herb-community change. Our study sites were not grazed by livestock and have not burned since Whittaker's time, and exotic species were too sparse to be detectable in our sampling. In the sites that were not clear-cut in the 1960s through the 1980s, there have been no other large-scale human disturbances. Increased shading as a result of forest succession, exacerbated by 20th-century fire suppression, is an important cause of change in forests at low to moderate elevations in the western United States (38). However, succession should have produced changes largely opposite to those we observed in lower montane forests, such as an increase in late-seral herb species or increases in traits associated with shadier conditions. Finally, random yearly fluctuations are unlikely to account for our results, because our study species are perennial herbs that are buffered by the presence of overstories (3), and resampling a subset of our plots revealed no significant interyear differences in herb cover.
Fagre et al. (12) argue that “It is particularly challenging to predict the effects of climatic variability in mountain ecosystems because the diverse topography, steep environmental gradients and ecological isolation of mountains result in high levels of biodiversity and endemism; this results in a complex intersection of physical and biological features that are typically beyond the capacity of existing empirical databases and models.” The Klamath-Siskiyou region is an outstanding example of these complexities, making it an ideal model system for better understanding ecological contingency in the effects of climate change. Our results demonstrate that the understanding of contingency can be improved through a careful consideration of ecological limiting factors, although empirical refinement also is necessary and can be accomplished via comparisons of historical and modern data across communities within complex landscapes.
Methods
Study System.
Our study sites are in the Siskiyou National Forest in the vicinity of Oregon Caves National Monument (42°06′N, 123°27′W). The Siskiyou Mountains are the northernmost range of the Klamath-Siskiyou region, one of the most species-rich forested regions in the United States (25–27). Lower montane forests (500–1,200 m above sea level) are dominated by Douglas fir (Pseudotsuga menziesii) mixed with evergreen hardwoods (e.g., Lithocarpus densiflorus, Quercus chrysolepis, Arbutus menziesii) at lower elevations and by white fir (Abies concolor) at higher elevations (25, 51). Transitional forests (1,200–1,500 m above sea level) are dominated by white fir, and upper montane to subalpine forests (1,500–2,200 m above sea level) are dominated by red fir (Abies magnifica) and mountain hemlock (Tsuga mertensiana). Typical understory herbs include many genera shared with Pacific Northwestern and even northeastern US forests (25, 31, 55–57).
Commercial clear-cut logging in the Siskiyou National Forest occurred mainly in the 1960s through the 1980s and at elevations <1,500 m (31, 51, 58). Clear-cutting sometimes was coupled with debris burning, planting of conifers, and the use of broadleaf herbicides. In one watershed in our study area, roughly 34% of the late-successional midmontane forest was logged in this period (31). The second-growth stands found today are characterized by dense stands of small trees with abundant shrubs and herbs in the understory; few if any forest species are lost in the first 4 decades following logging (31). Fires have been mostly suppressed in the region since the 1940s (51, 58–60), and the resulting uninterrupted succession can lead to smaller canopy openings and preferential regeneration of shade-tolerant trees such as P. menziesii and A. concolor (38, 59).
Spatially averaged climate data from multiple stations surrounding Oregon Caves National Monument, in the center of our study system, show that mean summer temperatures have increased ∼2 °C since 1948, but growing-season precipitation has not changed significantly (30). Although data are inadequate to compare trends at low and high elevations, temperature fluctuations are similar at low-elevation Cave Junction (elevation 390 m above sea level, records beginning in 1963) and high-elevation Bigelow Camp (elevation 1,561 m above sea level, records beginning in 1990). Snow-water equivalent (the product of depth and water content) has declined at Grayback Peak (elevation 1,829 m above sea level, records beginning in 1936) in the high-elevation portion of our study region (30).
Robert Whittaker's Data.
In 1949–1951, Robert Whittaker sampled vegetation at more than 400 sites in the study area in an effort to develop methods for describing community variation (25). He sampled near roads or trails for convenience, chose sites within predetermined classes of elevation and topography, and visited each site only once. He recorded the name of the road or trail, site elevation to the nearest 30 m (100 feet), slope to the nearest 5°, and aspect to the nearest 30°. At each site, he established a 50 × 20-m plot within which he placed twenty-five 1-m2 quadrats at alternate meters along the center line. He recorded numbers of tree and shrub individuals in the whole plot by species and recorded tree individuals as “small” [<38.1 cm diameter at breast height (dbh) for conifers and <20.3 cm dbh for hardwoods] or “large.” He estimated the percent of areal cover for each herb species as the number of quadrat corners it intercepted out of the 100 total quadrat corners in the plot.
Whittaker rated each site on a scale of 1 to 10 that he called the “topographic moisture gradient” to capture the plant-community variation that he believed was caused primarily by slope and aspect but with additional contributions from soil depth and wind exposure. Low values indicated communities on cool or “mesic” sites (e.g., mild to moderate north-facing slopes); high values indicated communities on warm or “xeric” sites (e.g., steep south-facing slopes). Sometimes, for unknown reasons, he used gradient lengths other than 10 (range, 6–11) and/or assigned more than one score to a site. We standardized his scores to a 0–1 scale and averaged them to arrive at one score for each site. We found that these standardized scores were correlated (r = 0.51, P < 0.001) with January insolation, a function of slope and aspect (61).
Most of Whittaker's lower-elevation sites (500–1,200 m above sea level) along Grayback and Sucker Roads were logged in the 1960s through the 1990s, but comparable sites on adjacent Caves Highway remained unlogged because of their proximity to public campgrounds and the Oregon Caves National Monument. All of Whittaker's sites at intermediate elevations (1,200–1,500 m above sea level) were logged, and none of his high-elevation sites (1,500–2,100 m above sea level) were logged. We used only the 500- to 1,200-m band, in which logged and unlogged sites could be compared, and the unlogged 1,500–2,100 m band.
We obtained Whittaker's data from the Cornell University Library's Rare Document Division and entered it into a database with tree and shrub counts by species, herb cover by species, and site locations (road, elevation, slope, and aspect).
Modern Data Collection.
In June and July, 2007–2009, we repeated Whittaker's sampling as closely as possible. Following the same road or trail, we used a global positioning system to arrive at sites that were within 15 m of Whittaker's recorded elevations and that matched his recorded slopes and aspects closely. We chose sites within relatively homogeneous vegetation, following his known practice (62). We sampled all the unlogged sites we could find within the two elevational bands (n = 55 unlogged lower-elevation sites and 68 high-elevation sites) and a set of 44 logged sites that did not differ in mean elevation from the unlogged lower-elevation sites. We deviated from Whittaker's technique in only two ways. First, we visited each site a second time in later summer to look for additional species, but we found few (i.e., second visits contributed only 1.6% of the total species observations in the study). Second, we recorded the dbh of each individual tree.
We saw occasional stumps in otherwise unlogged areas at low elevations, presumably the result of small-scale harvesting early in the 20th century (58), which Whittaker also would have encountered in 1949–1951. We avoided these stumps as much as possible in site selection, as we assume Whittaker did. We were unable to locate site-specific information on logging dates or treatments. However, a tight positive relationship exists between the age of a clear-cut stand and its mean tree dbh (r2 = 0.996, n = 6; 31). We therefore used the mean tree dbh of logged sites as a covariate in preliminary analyses, but we never found significant effects.
Plant and Community Traits.
SLA.
In June and July 2009, we used standard methods to measure SLA for all herb species (excluding saprophytic Corallorhiza spp.) that showed nonzero cover values in either Whittaker's dataset or ours. We collected one or two leaves from each of five mature and nonsenesced individuals, kept them hydrated in water tubes, photographed them, and measured their areas using image analysis software (63). We dried the leaves for 72 h at 60 °C, weighed them, and calculated SLA as area/dry mass (cm2/g) (34). In biomes around the world, SLA has been found to increase (and equivalently, its inverse, known as “leaf mass per area,” or LMA, to decrease) from hot, sunny, and/or dry environments to cool, shady, and/or moist environments, although SLA also varies within environments (32–34). SLA also is related consistently to other plant traits; for example, it is negatively correlated with leaf longevity (64). We calculated an abundance-weighted mean SLA for each plot by summing the product of each species’ percent of cover and its SLA. A high value indicates a community dominated by mesic (cool, moist)-adapted species.
Biogeographic affinity.
To classify species as northern or southern, we used Raven and Axelrod's monograph on the Californian flora (36). Northern species belong to families or genera that these authors classify as Arcto-Tertiary, meaning their ancestors are thought to have belonged to a mesic pan-temperate Tertiary flora. In contrast, southern species belong to families or genera that are of Madro-Tertiary (subtropical semiarid), Californian (Mediterranean climate), or warm temperate desert origin; other minor categories include “cosmopolitan” and “unknown.” Northern species are more prevalent in cooler, wetter, and shadier environments, and southern species show the reverse distributional patterns (36, 37, 44). We calculated the percent of cover of each community consisting of northern species. A high value again means a community dominated by mesic-adapted species.
Community similarity to warm topographic positions.
To measure the overall resemblance of any given herb community to communities found in warm (steep, southerly) versus cool (moderate, northerly) topographic microclimates, we used an ordination approach (also see 28). We ordinated the herb data using NMS ordination in PC-ORD version 4.14 (39), excluding species found in <5% of samples. We rotated axis 1 of the ordination to maximize its correlation with Whittaker's topographic moisture gradient, so that a low axis 1 score indicated a community in a mesic environment such as a moderate north-facing slope, and a high axis 1 score indicated a community in a warm environment such as a steep south-facing slope. Under a warming climate, we expect the community at any given site to show a higher axis 1 score in 2007–2009 than in 1949–1951, indicating that herb composition has shifted over time in the same direction that composition changes over space from mesic (cooler and moister) to xeric (warmer and drier) topographic microclimates. For each site we calculated the difference between its 1949–1951 and 2007–2009 axis 1 ordination scores. In this case, a high value means a community that has shifted to become more dominated by xeric-adapted species.
Late-seral indicator status.
To classify herbs as late-seral indicators, we searched the literature for statistical evidence that a species or genus was significantly affiliated to old primary forests in North America. We subsequently deleted five of the congeners based on expert recommendations. The following 20 late-seral indicators were identified: Achlys triphylla, Adenocaulon bicolor, Chimaphila menziesii, Chimaphila umbellata, Clintonia uniflora, Coptis laciniata, Corallorhiza maculata, Corallorhiza menziesii, Corallorhiza striata, Goodyera oblongifolia, Linnaea borealis, Listera cordata, Listera caurina, Osmorhiza berteroi, Phlox adsurgens, Pyrola asarifolia, Pyrola picta, Smilacina racemosa, Smilacina stellata, and Trillium ovatum (31, 53–57). We calculated the relative percent of cover by these late-seral indicator species for each plot. A high value identifies a community dominated by species characteristic of undisturbed late-successional forests.
Species origin.
To classify species as native or exotic, we used regional floras (65, 66) and the US Department of Agriculture PLANTS database (67).
Forest Cover Analyses.
We obtained aerial photographs of the study region from a 1940 US Forest Service inventory and from a modern study with 0.5-m resolution (41). We rectified the 1940 aerial photography using the georectification module in GRASS 6.4.0 RC5 (68). Because the flight tracks of the 1940 imagery did not overlap side-to-side, not all study sites were represented on the 1940 images, but we were able to identify the locations of 5 unlogged and 21 logged low-elevation sites and 44 high-elevation sites on the 1940 imagery. For each image we found 18 ground control points that spanned the entire extent of the image and could be identified on both the 1940 and the 2005 imagery. We used both a second- and third-order polynomial fit to rectify the images, choosing the rectified image that gave the best local fit on a site-by-site basis. We assessed percent of woody cover for each study site in a 120 × 150-m rectangle centered on the field-measured latitude and longitude of the site. We estimated cover to the nearest 10% but also included 5% and 95% as cover levels.
Statistical Analyses.
To analyze herb-community changes potentially related to climate, we used MANOVA with change in mean SLA, change in percent of northern species, and change in ordination axis 1 score as multiple response variables and community type (low elevation unlogged, low elevation logged, and high elevation) as the predictor variable. We used ANOVAs to compare changes in species richness and total herb cover between low- and high-elevation forests and corrected for multiple comparisons using a Dunn–Sidak correction (69).
To analyze changes potentially related to land use (logging, fire suppression), we asked if the change in relative abundance of late-seral indicator species over time was different from zero within each community type by using multiple t tests and correcting for multiple comparisons using the Dunn–Sidak correction (69). Similarly, to test whether the change in forest canopy cover was different from zero within each community type, we used multiple t tests and a Dunn–Sidak correction to determine whether the difference in canopy cover from aerial photos over time differed from zero. Finally, we used independent regressions to test whether changes in forest canopy cover were responsible for the observed differences in the abundance-weighted mean SLA, percent of northern species, and ordination axis 1 scores per plot.
Supplementary Material
Acknowledgments
L. L. Olsvig-Whittaker and the Cornell University Library's Division of Rare and Manuscript Collections provided access to Robert Whittaker's data. L. Barry, H. Cornell, T. Elder, K. Fuccillo, C. Garnier, J. Hansford, J. Hartman, A. Hollander, F. Hrusa, D. Inouye, E. Jules, M. Jules, K. Kostelnik, A. King, R. Mack, K. Moore, D. Odion, J. Orrock, R. Peet, J. Roth, M. Schwartz, A. Storfer, T. Talty, J. Thorne, M. Todd, K. Torres, G. Walter, T. Wentworth, the E.I.D. and S.H. laboratories, and two anonymous reviewers provided technical assistance and expert knowledge. Siskiyou Field Institute, Siskiyou National Forest, Oregon Caves National Monument, and the Marion Ownbey Herbarium at Washington State University provided logistical support. Funding was provided by National Science Foundation Grant DEB-0542451 and by the US Geological Survey Global Climate Change Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006823107/-/DCSupplemental.
References
- 1.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AE, Goulden ML. Rapid shifts in plant distribution with recent climate change. Proc Natl Acad Sci USA. 2008;105:11823–11826. doi: 10.1073/pnas.0802891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- 4.Moritz C, et al. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322:261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- 5.Peñuelas J, Boada M. A global change-induced biome shift in the Montseny mountains (NE Spain) Global Change Biology. 2003;9:131–140. [Google Scholar]
- 6.Grime JP, et al. The response of two contrasting limestone grasslands to simulated climate change. Science. 2000;289:762–765. doi: 10.1126/science.289.5480.762. [DOI] [PubMed] [Google Scholar]
- 7.Grime JP, et al. Long-term resistance to simulated climate change in an infertile grassland. Proc Natl Acad Sci USA. 2008;105:10028–10032. doi: 10.1073/pnas.0711567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klanderud K, Totland O. Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology. 2005;86:2047–2054. [Google Scholar]
- 9.Theurillat JP, Guisan A. Potential impact of climate change on vegetation in the European Alps: A review. Clim Change. 2001;50:77–109. [Google Scholar]
- 10.Loarie SR, et al. Climate change and the future of California's endemic flora. PLoS ONE. 2008;3:e2502. doi: 10.1371/journal.pone.0002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkett VR, et al. Nonlinear dynamics in ecosystem response to climatic change: Case studies and policy implications. Ecological Complexity. 2005;2:357–394. [Google Scholar]
- 12.Fagre DB, Peterson DL, Hessl AE. Taking the pulse of mountains: Ecosystem responses to climatic variability. Clim Change. 2003;59:263–282. [Google Scholar]
- 13.Wiens JA, Bachelet D. Matching the multiple scales of conservation with the multiple scales of climate change. Conserv Biol. 2010;24:51–62. doi: 10.1111/j.1523-1739.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 14.Grabherr G, Gottfried M, Pauli H. Climate effects on mountain plants. Nature. 1994;369:448. doi: 10.1038/369448a0. [DOI] [PubMed] [Google Scholar]
- 15.Parolo G, Rossi G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology. 2008;9:100–107. [Google Scholar]
- 16.Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology. 2007;13:147–156. [Google Scholar]
- 17.Adams HD, et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA. 2009;106:7063–7066. doi: 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breshears DD, et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban DL, Miller C, Halpin PN, Stephenson NL. Forest gradient response in Sierran landscapes: The physical template. Landscape Ecology. 2000;15:603–620. [Google Scholar]
- 20.Jolly WM, Dobbertin M, Zimmerman NE, Reichstein M. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys Res Lett. 2005;32:LI18409. [Google Scholar]
- 21.Crimmins TM, Crimmins MA, Bertelsen CD. Flowering range changes across an elevation gradient in response to warming summer temperatures. Global Change Biology. 2009;15:1141–1152. [Google Scholar]
- 22.Watson RT, Albritton DL. Climate Change 2001: Synthesis Report, Summary for Policymakers (Third IPCC Assessment Report). An Assessment of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- 23.Noss RF. Beyond Kyoto: Forest management in a time of rapid climate change. Conserv Biol. 2001;15:578–590. [Google Scholar]
- 24.Opdam P, Wascher D. Climate change meets habitat fragmentation: Linking landscape and biogeographical scale levels in research and conservation. Biol Conserv. 2004;117:285–297. [Google Scholar]
- 25.Whittaker R. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr. 1960;30:279–338. [Google Scholar]
- 26.Coleman RG, Kruckeberg AR. Geology and plant life of the Klamath-Siskiyou mountain region. Natural Areas Journal. 1999;19:320–340. [Google Scholar]
- 27.Ricketts TH, et al. Terrestrial Ecoregions of North America: A Conservation Assessment. Washington, D.C.: Island; 1999. [Google Scholar]
- 28.Damschen EI, Harrison SP, Grace JB. Climate change effects on an endemic-rich edaphic flora: Resurveying Robert H. Whittaker's Siskiyou sites (Oregon, USA) Ecology. 2010;91:3609–3619. doi: 10.1890/09-1057.1. [DOI] [PubMed] [Google Scholar]
- 29.NOAA . National Environmental Satellite, Data, and Information Service U.S. Climate Data. Washington, D.C.: National Oceanic and Atmospheric Administration; 2009. [Google Scholar]
- 30.PRISM Klamath Parks Data Viewer. 2010 [Google Scholar]
- 31.Jules MJ, Sawyer JO, Jules ES. Assessing the relationships between stand development and understory vegetation using a 420-year chronosequence. For Ecol Manage. 2008;255:2384–2393. [Google Scholar]
- 32.Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: Some leading dimensions of variation between species. Annu Rev Ecol Evol Syst. 2002;33:125–159. [Google Scholar]
- 33.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 34.Cornelissen JH, et al. A handbook for protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- 35.Ackerly DD. Evolution, origin and age of lineages in the Californian and Mediterranean floras. J Biogeogr. 2009;36:1221–1233. [Google Scholar]
- 36.Raven P, Axelrod D. Origin and Relationships of the California Flora. Berkeley, CA: Univ of California Publications in Botany; 1978. [Google Scholar]
- 37.Harrison S, Grace JB. Biogeographic affinity helps explain productivity-richness relationships at regional and local scales. Am Nat. 2007;170(Suppl 2):S5–S15. doi: 10.1086/519010. [DOI] [PubMed] [Google Scholar]
- 38.Skinner CN. Change in spatial characteristics of forest openings in the Klamath mountains of northwestern California, USA. Landscape Ecology. 1995;10:219–228. [Google Scholar]
- 39.McCune B, Mefford MJ. PC-ORD: Multivariate analysis of ecological data. Gleneden Beach, OR: MjM Software Design; 1999. Version 4.14. [Google Scholar]
- 40.McCune B, Grace JB. Analysis of Ecological Communities. Gleneden Beach, OR: MjM Software Design; 2002. [Google Scholar]
- 41.US Department of Agriulture . US Department of Agriculture Farm Service Agency, Aerial Photography Field Office. 2005. Josephine County, Oregon. Retrieved from Naip_1-1_1n_s_or033_2005_1.sid. Aerial photography. National Agriculture Imagery Program. [Google Scholar]
- 42.Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. Warming and earlier spring increase western U.S. forest wildfire activity. Science. 2006;313:940–943. doi: 10.1126/science.1128834. [DOI] [PubMed] [Google Scholar]
- 43.Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci. 2003;164:S165–S184. [Google Scholar]
- 44.Valiente-Banuet A, Rumebe AV, Verdú M, Callaway RM. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proc Natl Acad Sci USA. 2006;103:16812–16817. doi: 10.1073/pnas.0604933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robson TM, Rodríguez-Calcerrada J, Sánchez-Gómez D, Aranda I. Summer drought impedes beech seedling performance more in a sub-Mediterranean forest understory than in small gaps. Tree Physiol. 2009;29:249–259. doi: 10.1093/treephys/tpn023. [DOI] [PubMed] [Google Scholar]
- 46.Sang WG, Bai BF. Vascular diversity patterns of forest ecosystem before and after a 43-year interval under changing climate conditions in the Changbaishan Nature Reserve, northeastern China. Plant Ecology. 2009;201:115–130. [Google Scholar]
- 47.Suarez ML, Kitzberger T. Recruitment patterns following a severe drought: Long-term compositional shifts in Patagonian forests. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 2008;38:3002–3010. [Google Scholar]
- 48.Johnson DM, Smith WK. Cloud immersion alters microclimate, photosynthesis and water relations in Rhododendron catawbiense and Abies fraseri seedlings in the southern Appalachian Mountains, USA. Tree Physiol. 2008;28:385–392. doi: 10.1093/treephys/28.3.385. [DOI] [PubMed] [Google Scholar]
- 49.Mendoza I, Zamora R, Castro J. A seeding experiment for testing tree-community recruitment under variable environments: Implications for forest regeneration and conservation in Mediterranean habitats. Biol Conserv. 2009;142:1491–1499. [Google Scholar]
- 50.Erschbamer B, Kiebacher T, Mallaun M, Unterluggauer P. Short-term signals of climate change along an altitudinal gradient in the South Alps. Plant Ecol. 2009;202:79–89. [Google Scholar]
- 51.Agee JK. Fire history along an elevational gradient in the Siskiyou Mountains, Oregon. Northwest Science. 1991;65:188–199. [Google Scholar]
- 52.Inouye DW, Wielgolaski F-E. Phenology of high-altitude climates. In: Schwartz MD, editor. Phenology: An Integrative Environmental Science. New York: Springer; 2003. pp. 195–214. [Google Scholar]
- 53.Fleming RL, Baldwin KA. Effects of harvest intensity and aspect on a boreal transition tolerant hardwood forest. I. Initial postharvest understory composition. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 2008;38:685–697. [Google Scholar]
- 54.Burke DM, Elliott KA, Holmes SB, Bradley D. The effects of partial harvest on the understory vegetation of southern Ontario woodlands. For Ecol Manage. 2008;255:2204–2212. [Google Scholar]
- 55.Halpern CB, McKenzie D, Evans SA, Maguire DA. Initial responses of forest understories to varying levels and patterns of green-tree retention. Ecol Appl. 2005;15:175–195. [Google Scholar]
- 56.D'Amato AW, Orwig DA, Foster DR. Understory vegetation in old-growth and second-growth Tsuga canadensis forests in western Massachusetts. For Ecol Manage. 2009;257:1043–1052. [Google Scholar]
- 57.Moola FM, Vasseur L. Recovery of late-seral vascular plants in a chronosequence of post-clearcut forest stands in coastal Nova Scotia, Canada. Plant Ecology. 2004;172:183–197. [Google Scholar]
- 58.McKinley G, Frank D. Stories on the Land: An Environmental History of the Applegate and Upper Illinois Valleys. Report to the Medford District, U.S. Bureau of Land Management. Medford, OR: U.S. Bureau of Land Management; 1995. [Google Scholar]
- 59.Taylor AH, Skinner CN. Spatial patterns and controls on historical fire regimes and forest structure in the Klamath Mountains. Ecol Appl. 2003;13:704–719. [Google Scholar]
- 60.Odion DC, et al. Patterns of fire severity and forest conditions in the western Klamath Mountains, California. Conserv Biol. 2004;18:927–936. [Google Scholar]
- 61.McCune B. Improved estimates of incident radiation and heat load using non-parametric regression against topographic variables. Journal of Vegetation Science. 2007;18:751–754. [Google Scholar]
- 62.Westman WE, Peet RK. Whittaker, Robert H. (1920–1980)—the man and his work. Vegetatio. 1982;48:97–122. [Google Scholar]
- 63.Rasband WS. (1997-2009) ImageJ . Bethesda, MD: National Institutes of Health; [Google Scholar]
- 64.Westoby M. Choosing species to study. Trends Ecol Evol. 2002;17:587. [Google Scholar]
- 65.Kozloff EN. Plants of Western Oregon, Washington, and British Columbia. Portland, OR: Timber; 2005. [Google Scholar]
- 66.Hickman JC. The Jepson Manual. Berkeley, CA: Univ of California Press; 1993. [Google Scholar]
- 67.US Department of Agriculture . The PLANTS Database, Version 3.5. Baton Rouge, LA: National Plant Data Center; 2008. [Google Scholar]
- 68.Neteler M, Mitasova H. Open Source GIS: A GRASS GIS Approach. 3rd Ed. New York: Springer; 2007. [Google Scholar]
- 69.Sokal RR, Rohlf FJ. Biometry. 3rd Ed. New York: W.H. Freeman and Company; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.