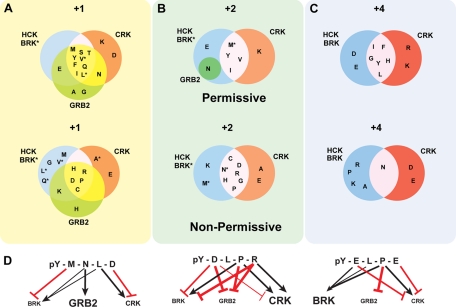
Fig. 5.
Combination of permissive and non-permissive residues at various positions creates a complex linguistics that underlies highly selective binding decisions. A–C, Venn diagrams represent the regions of overlap and exclusivity among permissive and non-permissive residues for the SH2 domains of Crk, Grb2, Hck, and Brk at the positions +1, +2, and +4. The +3 position is not represented as this was a fixed permissive position for Brk, Crk, and Hck. Only partial data are shown for Grb2 both for simplicity and because the Grb2 non-permissive residues were mapped on the basis of fixing +2 Asn and thus have only partial overlap in the mapping of non-permissive residues. Raw OPAL arrays on which these are based are shown in Fig. 4 and supplemental Fig. S1. Specific differences between Hck and Brk are indicated by an asterisk. D, conceptualization of the factors contributing to discrete binding decisions for Brk, Grb2, and Crk SH2 domains. Three peptides, pYMNLD, pYDLPR, and pYELPE, are shown that conform to the consensus (pYXX(P/L)) for Crk SH2 binding. The positive and negative contributions of each residue that determine array-positive binding to Grb2, Crk, and Brk, respectively, are illustrated. Black arrows indicate additional permissive residues, whereas red lines indicate non-permissive residues. The weight of the line indicates the relative intensity of these factors. The text size for each SH2 domain indicates the preference for that particular peptide to a particular SH2 domain (large text, stronger preference; small text, weak preference).