Abstract
Selenium is an essential micronutrient that functions as an antioxidant. Yet, at higher concentrations, selenium is pro-oxidant and toxic. In extreme cases, exposures to excess selenium can lead to death or selenosis, a syndrome characterized by teeth, hair and nail loss, and nervous system alterations. Recent interest in selenium as an anti- tumorigenic agent has reemphasized the need to understand the mechanisms underlying the cellular consequences of increased selenium exposure. We show here, that in the nematode, Caenorhabditis elegans, selenium has a concentration range in which it functions as an antioxidant, but beyond this range it exhibits a dose- and time-dependent lethality. Oxidation-induced fluorescence emitted by the dye, carboxy-H2DCFDA, indicative of reactive oxygen species formation was significantly higher in animals after a brief exposure to 5mM sodium selenite. Longer-term exposures lead to a progressive selenium-induced motility impairment that could be partially prevented by coincident exposure to the cellular antioxidant–reduced glutathione. The C elegans glrx-21 gene belongs to the family of glutaredoxins (glutathione-dependent oxidoreductases) and the glrx-21(tm2921) allele is a null mutation that renders animals hypersensitive for the selenium-induced motility impairment, but not lethality. In addition, the lethality of animals with the tm2921 mutation exposed to selenium was unaffected by the addition of reduced glutathione, suggesting that GLRX-21 is required for glutathione to moderate this selenium-induced lethality. Our findings provide the first description of selenium-induced toxicity in C elegans and support its use as a model for elucidating the mechanisms of selenium toxicity.
Keywords: selenium, antioxidant, toxicity, glutaredoxin, glutathione, glrx-21
Selenium (Se) is an essential micronutrient required for antioxidant activity and for normal endocrine and immune system function. Rare selenium deficiencies are reported to cause an irreversible cardiomyopathy (Keshans disease), preventable with selenium supplementation (Ge and Yang, 1993). Whereas the recommended daily allowance of 50–200 μg of Se is considered to be beneficial, ingestions of > 800 μg/day can lead to death (See et al., 2006) or to selenosis, a syndrome characterized by hair and nail loss, loose teeth, hepatotoxicity, a demyelinating peripheral neuropathy, and in some cases motor neuron disease clinically indistinguishable from amyotrophic lateral sclerosis or polio (Boosalis, 2008; Kilness and Hichberg, 1977; Vinceti et al., 1996, 2001; Yang et al., 1983). Similar neurological symptoms associated with increased mortality were observed in pigs that had consumed selenium-accumulating range plants (e.g., Astragalus bisulcatus) or food supplemented with selenium (Panter et al., 1996; Wilson et al., 1983), and in other domestic and laboratory animals (Ammar and Couri, 1981; MacDonald et al., 1981; Tiwary et al., 2006), although mortality varied with the type and source of selenium, as well as the species exposed (Lenz and Lens, 2009).
Excess selenium has been shown to accumulate as selenomethionine (SeMet) in proteins of hair and hooves/nails, in tissues including heart, liver, muscle, and skin, and to affect regions of the central nervous system (Kim and Mahan, 2001; Panter et al., 1996; Tiwary et al., 2006; Yang et al., 1983). The substitution of SeMet for methionine is known to alter protein stability (Jackson and Combs, 2008) and was recently demonstrated to affect target protein binding of the calcium regulatory protein, calmodulin (Yamniuk et al., 2009). Selenocysteine can be generated from SeMet and is site specifically incorporated into selenoproteins, such as thioredoxin reductases (TRXR) and glutathione peroxidases (GPX), proteins that function as enzymatic antioxidants (Battin and Brumaghim, 2009; Lu et al., 2009). The GPX proteins reduce hydrogen peroxides and lipid hydroperoxides, an action leading to the oxidation of glutathione (GSH), a cofactor for this reaction, which also functions as the major intracellular antioxidant (Forman et al., 2009). Depletion of intracellular pools of GSH during selenium-induced oxidative stress is furthered by the formation of hydrogen selenide, a product of selenium metabolism highly reactive with GSH and other intracellular thiols. This loss of GSH antioxidant activity is believed to allow damaging reactive oxygen species (ROS) levels to rise in cells and has been proposed as one of the mechanisms contributing to the overall oxidative damage and cell death observed with toxic selenium exposures (Misra and Niyogi, 2009; Spallholz and Hoffman, 2002).
The glutaredoxins (GLRX or GRX) are a group of oxidoreductases that catalyze the reversible reduction of protein disulfides through the cysteinyl residues of their active site (Ghezzi and Di Simplicio, 2009) and are in turn maintained in their reduced active form through the nonenzymatic oxidization of GSH (Lillig et al., 2008). GLRXs are classified into two major groups: dithiol or monothiol based on the consensus sequences found within their active sites (CPYC and CGFS, respectively) (Lillig et al., 2008). Deletion of both yeast dithiol GLRXs led to a selenium-induced growth inhibition that was reversible in an oxygen-free environment (Lewinska and Bartosz, 2008) and more recently shown to protect against selenium-induced cell death (Izquierdo et al., 2010). In contrast, small-interfering RNA knockdown of human GRX-1 decreased selenium-induced cytotoxicity in a human lung cancer cell line (Wallenberg et al., 2010). These studies suggest GLRX proteins play an essential role in selenium-induced toxicity.
Recent advances in understanding the effects of both acute high-dose and long-term low-dose exposures to selenium have been sparked by interest in selenium as an anti-tumorigenic agent (Batist et al., 1986; Facompre and El-Bayoumy, 2009; Jackson and Combs, 2008). This interest coupled with the commercialization of selenium because of its antioxidant capabilities has led to increased supplementation of selenium in food products (Boosalis, 2008; Navarro-Alarcon and Cabrera-Vique, 2008). In addition, large amounts of selenium are released into the atmosphere through mining operations, processing, and consumption of fossil fuel, and into the soil through agricultural irrigation in areas with high selenium content (Lemly, 1997; Lenz and Lens, 2009; Palmer et al., 2010). These factors have made elucidating the mechanisms of selenium toxicity more pressing as the sum of their effects increases the overall risk that a toxic selenium exposure will occur. The aim of this study was to evaluate the effects of selenium exposure on both lethality and movement behaviors in the nematode Caenorhabditis elegans and to determine the role of the worm glutaredoxin system, exemplified by the gene glrx-21, in selenium-induced toxicosis.
MATERIALS AND METHODS
Strains, Maintenance, and Growth Conditions
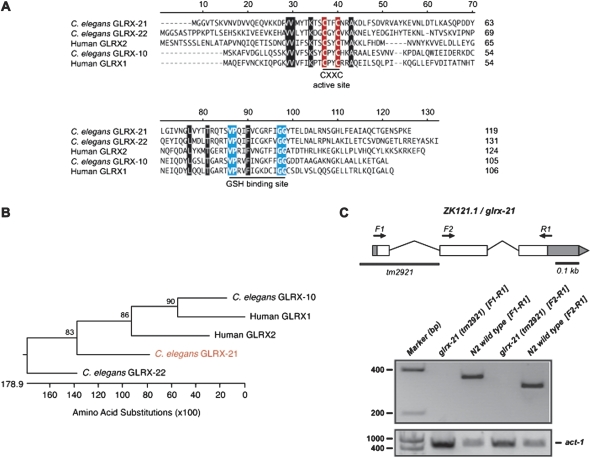
The C elegans N2 Bristol strain was used in all experiments requiring wild-type animals. In addition, the strain VZ54 glrx-21(tm2921)IV, which contains a 324 bp deletion of the glrx-21 gene that removes the proximal promoter and the first exon including the ATG codon, was used (Figure 3C). Six times backcrossed homozygous tm2921 deletion mutant animals were viable and phenotypically wild type.
FIG. 3.
The Caenorhabditis elegans glutaredoxin, GLRX-21 is most similar to human GLRX2. (A) Sequence alignment of all human and C elegans dithiol glutaredoxins. Compared with human GLRX2, GLRX-21 is 39% identical, whereas GLRX-22 has only 26.3% identity. The sequence used for human GLRX2 corresponds to the common domain for both the mitochondrial and nuclear isoforms (Lundberg et al., 2001). The cysteine residues at the redox active site are highlighted in red and those essential for GSH binding are in blue (Lillig et al. 2008). Other conserved residues in all the glutaredoxins are highlighted in black. The numbers on the right indicate the number of amino acid residues of each protein. (B) Phylogenetic tree of all human and C elegans dithiol glutaredoxins. The percentage bootstrap values (based on 1000 replications) are given on the nodes of the tree. Caenorhabditis elegans GLRX-21 is highlighted in red. (C) The glrx-21 gene is organized into three exons. Open boxes indicate the ORF, whereas the gray boxes designate the 5′-untranslated region (UTR) and 3′-UTR, respectively. The tm2921 deletion removes part of the proximal promoter plus the first exon and the first intron of the glrx-21 gene. Primers for RT-PCR were designed at the ATG codon and the beginning of the second exon (F1 and F2, respectively) and at the STOP codon (R1), respectively. The glrx-21 cDNA is only detected in the N2 wild-type lanes demonstrating that tm2921 is a null allele. act-1 primers were used for cDNA synthesis control.
Unless otherwise noted, maintenance and growth of animals was under standard conditions (Brenner, 1974) on modified (without added Ca2+; Estevez et al., 2004) nematode growth medium (NGM) agar plates containing as a food source a lawn of the bacterial strain E. coli OP50 (an uracil auxotroph described by Brenner, 1974). Because initial studies determined that increased temperature (25°C) enhanced the toxic effects of selenium exposure (Supplementary table 1), all studies were carried out at 20°C.
Reagents
The selenium used in this study was either sodium selenite (Na2SeO3; Spectrum, Gardena, CA) or seleno-l-methionine (SeMet; Sigma, St Louis, MO; Spectrum). Hydrogen peroxide (H2O2) was diluted from a 30% stock solution (Fisher Scientific, Fair Lawn, NJ). All reagents were dissolved in distilled water and either used in the assay as a liquid solution (lethality assays) or added to agar plates (behavioral assay and ROS detection) to the final concentrations described below.
Developmentally Synchronizing Adults
Populations of developmentally synchronized adults were isolated as described (Stiernagle, 1999) by first treating unsynchronized adult animals with a solution of bleach and sodium hydroxide to release their eggs. The eggs were placed in tubes containing liquid media (M9) without food and gently rocked at room temperature overnight. Animals hatched overnight without food will arrest in the first larval stage (L1) and will not molt into the next developmental stage (L2), until supplemented with food; thus, they become developmentally synchronized as starved L1 larvae. The starved L1 larvae were placed onto agar plates seeded with bacteria (initial growth plates) and allowed to develop until the last larval stage, L4. L4 animals were individually picked from these initial growth plates to fresh seeded agar plates (secondary growth plates) and allowed to develop 24 h further to the adult stage. These synchronized adults were used for the lethality and behavioral assays described below.
Lethality Assays
Synchronized adult animals were individually picked from their secondary growth plates and placed into 10 mm plates (liquid assay plates) containing 2 ml of a solution (concentrations ranged from 10−3 to 10mM, unless specifically indicated) of either sodium selenite (Na2SeO3), hydrogen peroxide (H2O2), or a combination of 1mM H2O2 and Na2SeO3, 2mM H2O2 and Na2SeO3, or 5mM Na2SeO3 and GSH. Controls in which animals were placed in dH2O only (0mM) were included with each experimental set. Animals in liquid assay plates were exposed under starvation conditions as they were not supplemented with OP50. An average of 10 animals was placed into the liquid on each plate and incubated at 20°C. After 6- or 12-h exposure, the number of living versus dead animals was scored. Animals were presumed to be dead if they did not initiate any movement in response to a harsh tap to the body with a platinum wire nor exhibit pharyngeal pumping as observed under high power magnification using a Leica MZ12s (Leica Microsystems Inc., Bannockburn, IL) or an Olympus SZX9 (Olympus America Inc., Center Valley, PA) dissecting microscope. After initial studies showed that 6-h exposure to Na2SeO3 resulted in a lethality rate of ≤ 20% for all the concentrations tested (Supplementary fig. 1), all subsequent liquid assays were measured for lethality after 12 h of exposure to Na2SeO3.
Animals were not observed to exhibit symptoms of hypoosmotic stress (such as swelling and bursting) after 12 h in dH2O in our liquid assays. This was similar to a study by Luke et al. (2007) that described wild-type animals as “mostly viable” after as much as 24 h in water. In addition, there was no measurable difference between the lethality rates for wild-type N2 animals exposed to dH2O alone versus those exposed to M9 (a minimal salt solution for C elegans growth; Brenner, 1974) in our liquid assay (n > 200 adult animals for each condition; no significance [p = 0.79] was found by ANOVA analyses compared with Tukey post-tests and with ANOVA analyses from SPSS v16).
Behavioral Assays
For the initial studies, plates containing 10 ml of agar that had previously been seeded with bacteria had 0.5 ml of either a Na2SeO3 (Fig. 4A) or SeMet (Supplementary fig. 2A) stock solution added such that the final concentrations in the agar were as listed (0.5, 1, 5, 10, 20mM). Control plates (0mM) to which 0.5 ml of carrier solution (dH20) was added were included in all behavioral assays. A final concentration of 5mM Na2SeO3 per agar plate was used in all additional studies beyond the initial assays (Figs. 5B and 5C, 6 and 7); this concentration and source of selenium was chosen because it produced a standard dose-response curve in which the animals showed a time-dependent decrease in the percentage of “motile” animals within each given population studied over the course of the 96-h exposure period (Figure 5A).
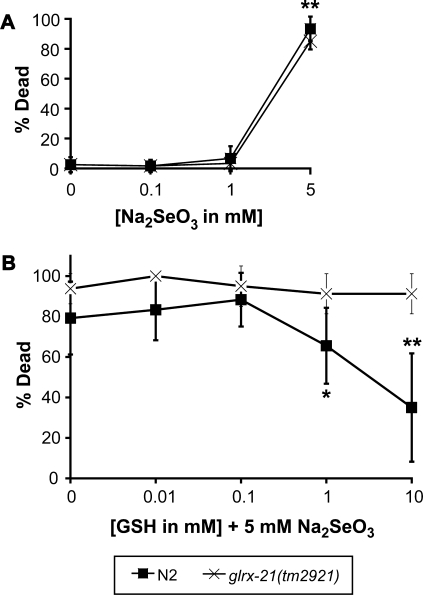
FIG. 4.
Selenium-induced lethality of a glutaredoxin mutant is not decreased by GSH. Animals were scored for lethality after 12-h exposure to (A) 5mM Na2SeO3 or (B) both 5mM Na2SeO3 and increasing concentrations of GSH (0–10mM). (A) Comparison of the selenium-induced lethality of the wild-type strain N2 (▪), and the glutaredoxin mutant strain, glrx-21(tm2921) (x). Each data point represents the averages of four to six plates with 10 animals per plate and is presented as the mean percentage of dead animals ± SD. ** p < 0.001, for wild-type and the glrx-21 mutant strain exposed to 5mM Na2SeO3 and compared across all other concentrations of Na2SeO3 (one-way ANOVA). Note. There is no statistically significant difference in lethality between wild-type and the glrx-21 mutant strain when compared within each concentration of Na2SeO3 (Student’s t-test) and across all concentrations except 5mM Na2SeO3 (one-way ANOVA). (B) The effects of reduced GSH on the selenium-induced lethality of the wild-type strain N2 (▪), and the glutaredoxin mutant strain, glrx-21(tm2921) (x). Each data point represents the averages of 4–13 plates with 10 animals per plate and is presented as the mean percentage of dead animals ± SD. Note. There is no statistically significant difference in lethality for the glrx-21 mutant strain when compared across all concentrations of GSH (0-10mM) tested (one-way ANOVA). *p < 0.05, compared with all concentrations of GSH (0.01–10mM) exposed wild-type animals and glrx-21 mutant animals, as well as the control (0mM GSH) for the glrx-21 mutant strain; **p < 0.01, compared across all concentrations of GSH (0.01–10mM) and controls for both the wild-type and glrx-21 mutant strains.
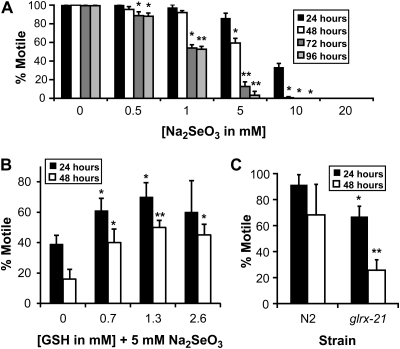
FIG. 5.
Sodium selenite reduces motile behavior. (A) Populations of adult animals were exposed to increasing concentrations (0–20mM) of sodium selenite (Na2SeO3) on agar plates. Motile behavior or the ability to move in any direction was scored in individual animals at 24-h intervals over the course of four days (96 h). All datasets represent the averages of three plates with 50 worms per plate and are presented as the mean percentage motility ± SD. *p < 0.05 compared with the 24-h time point at the same concentration and with the matched time point at 0mM Na2SeO3. ** p < 0.001, compared with the 24-h time point at the same concentration and with the matched time point at 0mM Na2SeO3 (B) Adult animals exposed on agar plates to 5mM Na2SeO3 with 0.7, 1.3, or 2.6mM reduced GSH were scored at 24-h intervals and compared with their selenium-only controls (0mM GSH) for their motility behavior. All datasets represent the averages of three to five plates with 20 animals per plate and are presented as the mean percentage motility ± SD. *p < 0.05, compared with 0mM GSH at the same time point. **p < 0.001, compared with 0mM GSH at the same time point. (C) Populations of adult animals of the strains N2 (wild type) and VZ54 glrx-21(tm2921) were exposed to 5mM Na2SeO3 and tested for their motile ability after 24 and 48 h of exposure on agar plates. All datasets represent the averages of three to four plates with 20 animals per plate and are presented as the mean percentage motility ± SD. *p < 0.05, compared with wild type at 24 h. **p < 0.001, compared with the glrx-21 mutant strain at 24 h and wild type at 24 and 48 h.
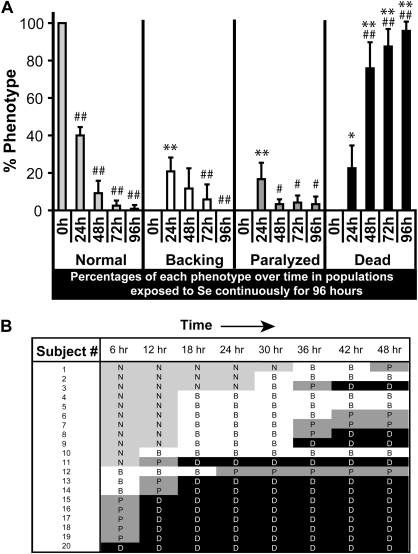
FIG. 6.
Chronic exposure to selenium results in a progression of behavioral phenotypes leading to lethality. (A) Populations of adult animals placed continuously on NGM agar plates supplemented with 5mM Na2SeO3 were scored at 24-h intervals to determine the percentages of animals with each behavioral phenotype (normal, backing [impaired], paralyzed) and for lethality (dead). The percentages of all phenotypes for each time point (0, 24, 48, 72, and 96 h) equal 100%. Each dataset represents six plates with 20 animals per plate and is presented as the mean percentage of animals with each phenotype ± SD. * p < 0.05, compared with normal at the same time point. **p < 0.001, compared with normal at the same time point. # p < 0.05, compared with 24-h within phenotype. ## p < 0.001, compared with 24-h within phenotype. (B) Individual animals were grown singularly on 5mM Na2SeO3 plates for a total of 48 h. The behavioral phenotype of each individual animal was assessed every 6 h. A total of 20 individual animals were observed. N = normal (light gray), B = backing (white), P = paralyzed (dark gray), D = dead (black).
FIG. 7.
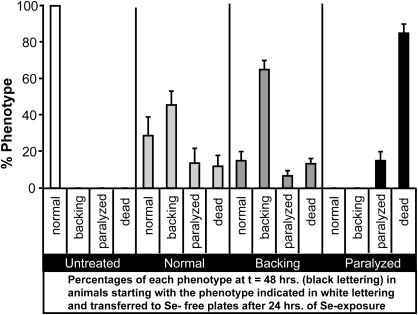
Behavioral deficits induced by acute selenium exposure are partially reversible. The behavioral phenotype of each adult animal was observed after growth for 24 h (normal, backing, paralyzed; white letters) on NGM plates supplemented with 5mM Na2SeO3. These animals were sorted by these initial behavioral phenotypes onto fresh NGM agar plates with no added selenium. After 24 h without selenium exposure, individual animals were again scored for their behavioral phenotype and for lethality (normal, backing, paralyzed, dead; black letters). Twenty animals per phenotype were placed on to each of three selenium-free plates for a total of 60 animals for each 24-h phenotype used. The phenotypes of animals grown for 48 h without selenium served as a control (untreated). Each graph bar represents the average population of animals with the 48-h phenotype (as indicated on the graph) and is depicted as the mean percentage ± SD.
The behavioral assays were performed by viewing individual animals under a Leica MZ12s (Leica Microsystems Inc.) dissecting microscope. Individual animals were tested for backward movement by tapping them on the head and observing their movement for 5 s to determine if they completed one sinusoidal wave backward within this time period. If they did not complete this task as specified, they were scored as “backing” defective. Both “backing” defective and normal backing animals were tested for their ability to move forward by tapping on the tail and observing for 5 s to see if they completed one sinusoidal wave forward. Animals that did not complete both the backing and forward movement tests were scored as “paralyzed,” whereas those animals that completed both tests were scored as “normal.” After observing animals individually under the microscope, those that failed the movement tests and when viewed under higher magnification (×100) showed no pharyngeal pumping, were scored as “dead.” The term “motile” excludes all animals that were determined to be “paralyzed” or ”dead” but includes animals that moved normally (normal) or failed to back (backing). Data were reported as the average percentage of animals within the population studied that expressed the specified phenotype (normal, backing, paralyzed, dead, or motile), except when individual animal data are reported.
Chronic Studies
Population.
Populations of animals with continuous exposure to selenium were examined over time by placing developmentally synchronized adult animals on agar plates containing the indicated concentrations (0, 0.5, 1, 5, 10, and 20mM) and sources of selenium (Na2SeO3 or SeMet). About 20–50 animals were placed on each agar plate. Where the sources and concentrations of selenium were not indicated on the graph, 5mM Na2SeO3 was used. Datasets were collected at intervals of 24 h (24, 48, 72, and 96 h) for population studies by assaying the behavior (motile or normal, backing, paralyzed, and dead) of each individual animal as described above.
Individual.
Our individual study of chronic selenium exposure (Figure 6B) included a total of 20 animals that were each scored for their behavioral phenotype (normal, backing defective, paralyzed, or dead) at intervals of 6 h (6, 12, 18, 24, 30, 36, 42, and 48). Each plate included just one animal, which was characterized individually over the 48-h period.
Acute Studies
In order to examine the delayed effects of short-term exposure to selenium, 200 developmentally synchronized adult animals were transferred to each of three individual plates containing bacteria and 5mM Na2SeO3 (treated: total of 600 animals) and to one control plate with bacteria but no added selenium (untreated: total of 200 animals). After 24 h, individual behavioral phenotypes (normal, backing defective, paralyzed, or dead) were assayed as described above, and 20 animals of each phenotype (normal, backing, and paralyzed) were removed from each of the Na2SeO3 treated plates. These animals were placed onto selenium-free plates; three plates for each of the 24-h phenotypes were used. All animals from the control plates were assayed as normal. Twenty normal untreated animals were placed on to each of three selenium-free plates as controls. All animals were allowed to grow for an additional 24 h on the Se-free plates and then assayed again for their behavioral phenotypes (Figure 7).
Growth on Defined Liquid Medium
Adult animals were grown in the defined liquid media, CeMM as described (Szewczyk et al., 2003) without the added presence of bacteria under otherwise standard growth conditions. One hundred adult animals were placed in CeMM with or without the addition of 5mM Na2SeO3 for 24 h and then observed to determine the percentage of motile animals. Data were analyzed from four sets of experiments for a total of 400 animals measured for each condition.
Reduced glutathione (GSH) treatment
Reduced glutathione (GSH; l-glutathione reduced) was purchased from Sigma-Aldrich and dissolved in distilled water for all assays. For lethality, GSH was dissolved in distilled water and added as a liquid to each assay plate; for behavioral assays and ROS detection (below) GSH was added along with Na2SeO3 to agar plates. All plates (liquid and agar) contained a final concentration of 5mM Na2SeO3 and the indicated concentration of GSH.
ROS Detection
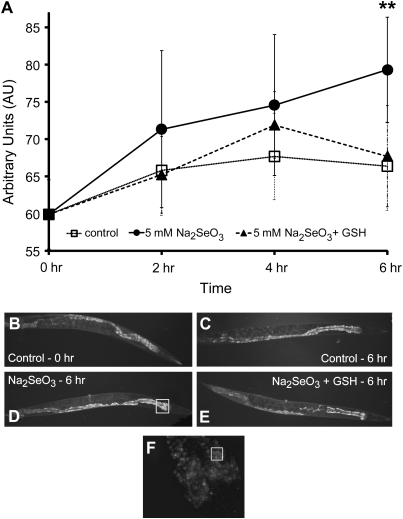
Adult N2 wild-type animals were exposed for 2, 4, or 6 h with or without 5mM Na2SeO3, or with 5mM Na2SeO3 and 10mM GSH, on agar plates as described above for the behavioral assays. To detect changes in the levels of ROS, a stock solution of 20mM carboxy-H2DCFDA [5-(and-6)-carboxy-2′,7’-dichlorodihydrofluorescein diacetate–mixed isomers; Molecular Probes, Eugene, OR) in dimethyl sulfoxide was diluted to 20 μM in M9 buffer. Five 100-μl drops of the diluted dye were placed onto 10 mm plates that were contained inside a humid chamber to prevent desiccation during the 3-h incubation period. Ten animals were individually picked from their exposure plates into each of the five drops following the exposure periods with one plate per exposure type/time point. Animals used to detect the 0-h time point were moved directly from their secondary growth plates into the dye. After the staining incubation period, individual animals were picked from the dye onto a slide for viewing. Animals that appeared dead or damaged (i.e., could not swim in the drop or that “exploded” after being placed on the slide) were eliminated from further analysis. Individual animals were visualized on an Olympus BX51 microscope equipped with fluorescence optics (Olympus America Inc.) utilizing a fluorescein isothiocyanate filter and documented by a QImaging Retiga 1300 camera and imaging system (QImaging, Surrey, British Columbia, Canada). A total of 10–13 images per exposure type/time period were imported into Adobe Photoshop (Adobe Systems Inc., San Jose, CA) for analysis. Each image was magnified to 600% of the original image (using the Photoshop magnification tool), and the luminosity levels were collected from the five brightest 100 pixel dense regions of the gut located immediately behind the second pharyngeal bulb (Fig. 2F for example). The average luminosity for all five regions was calculated per animal. The averages of all the animals per exposure type/time point were calculated and expressed in arbitrary units (±SD) on the graph depicted in Figure 2A.
FIG. 2.
Selenium toxicity is induced by free radical formation. (A) The average luminosities of adult N2 wild-type animals exposed on agar plates for 2, 4, or 6 h to either 5mM Na2SeO3 (•) or 5mM Na2SeO3 and 10mM GSH (▴) followed by a 3-h incubation with the dye carboxy-H2DCFDA were compared with control (□) animals mock exposed on plates for 0, 2, 4, or 6 h and then incubated for 3 h with dye. Animals that were dead or dying were eliminated before fluorescence measurements were taken as described in the “Materials and Methods” section. Each data point displayed on the graph represents the average luminosity from 10–13 animals obtained by measuring the five brightest 100 pixel dense regions of the gut located immediately posterior to the head and is represented by arbitrary units ± SD. **p < 0.001, compared with the 6-h time point for both the control and Na2SeO3 + GSH. (B–E) Representative photomicrographs of control (mock exposed) animals at 0 h (B) and 6 h (C) are shown for comparison to animals exposed for 6 h to either 5mM Na2SeO3 [(D)—box indicates region magnified in (F)] or 5mM Na2SeO3 and 10mM GSH (E). (F) A region of the 5mM Na2SeO3 exposed animal shown in (D) was magnified to 600%. The open box is representative of a 100 pixel dense regions in which the luminosity was measured to generate the graph (A).
Sequence Alignment and Phylogenetic Analysis
The protein sequences were aligned using the ClustalW program (Thompson et al., 1994). The phylogenetic analysis was produced by applying the neighbor-joining method of Saitou and Nei (1987) to the alignment data. Both the ClustalW alignment and neighbor-joining algorithm were implemented by using the Megalign program included in the DNASTAR Software Package (DNASTAR, Madison, WI). Statistical support for nodes of the neighbor-joining trees was assessed by using the 50% majority rule consensus trees compiled from 1000 bootstrap replications (Felsenstein, 1981) implemented with the NJplot Program (http://pbil.univ-lyon1.fr/software/njplot.html).
RT-PCR
Total RNA from N2 wild-type and VZ54, glrx-21(tm2921) was isolated with Trizol Reagent (Invitrogen, Carlsbad, CA) and subsequently DNaseI digested. Complementary DNA (cDNA) synthesis was performed using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA) following manufacturer instructions. Amplification was performed with about 1 ng cDNA. The primers used for the reverse transcriptase (RT)-PCR were the following:
glrx-21 F1: 5′- atgggaggagtcacctcaaaag-3′
glrx-21 F2: 5′- ggatccagttgtgatgtacac-3′
glrx-21 R1: 5- ttctttcggcgaattctctcc-3.
The amplification was done for 35 cycles (95° for 1 min, 50° for 1 min, 72° for 2 min). cDNA synthesis was monitored using act-1 primers:
act-1 F1: 5′- gaggcccaatccaagaga-3′
act-1 R1: 5′- tgttggaaggtggagagg-3′.
Statistical Analysis
Statistical analysis was initially performed using Microsoft Excel software. The means and SD reported were determined by averaging data obtained from all the plates of each strain or population type (e.g., treated or untreated) counted. Probability values were determined by applying the two-tailed Student’s t-test. Graphs were initially drawn with Excel and were prepared for publication using Adobe Illustrator (Adobe Systems Inc.). One-way ANOVA analyses were carried out using Bonferroni post-tests performed on GraphPad Prism Software v 5.02.4 (GraphPad Software Inc., La Jolla, CA).
RESULTS
Selenium is Both Toxic and Protective to C elegans
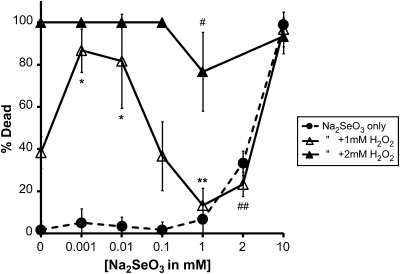
Selenium is a required micronutrient that has been highly publicized for its antioxidant activity and its potential role to both treat and prevent diseases (Facompre and El-Bayoumy, 2009; Reeves and Hoffmann, 2009). Yet, chronic exposures to doses as low as 400 μg/day (the upper limit for consumption) may induce toxicity in sensitive individuals (Lemly, 1997). To test whether selenium is toxic to C elegans, adult N2 wild-type animals were exposed to various nominal concentrations of sodium selenite (Na2SeO3) diluted in dH2O and then scored after 12 h at 20°C to determine the percentage of dead animals per plate (lethality assay). Under these conditions, selenium exhibited a dose-dependent increase in lethality that was most apparent with exposures above 1mM (Figure 1).
FIG. 1.
Selenium is both protective and toxic to Caenorhabditis elegans. The effects on the survival of wild-type animals exposed to increasing Na2SeO3 concentrations (0–10mM) either alone (•) or in the presence of 1mM (Δ) or 2mM (▴) H2O2. Each dataset represents the averages of six to nine plates with 10 animals per plate exposed in liquid for 12 h and is presented as the mean percentage of dead animals ± SD. The LC50 for Na2SeO3 exposure at 12 h was calculated to be 3.47mM. #p < 0.05, compared with 2mM H2O2 and 1mM Na2SeO3; ##p < 0.05, compared with 1mM H2O2 (no significant difference to 2mM Na2SeO3); * p < 0.01, compared with both 1mM H2O2 and either 0.001 or 0.01mM Na2SeO3; **p < 0.001, compared with 1mM H2O2 (no significant difference to 1mM Na2SeO3).
Hydrogen peroxide (H2O2) has been shown to be an oxidative stress–inducing agent in C elegans (Kumsta et al., 2010; Larsen, 1993). Under the conditions of our lethality assay (12 h at 20°C), adult N2 animals exposed to 1mM H2O2 died at a rate of 38.3 (±7.53)%, whereas concentrations of 2mM (Figure 1) and greater (data not shown) resulted in 100 (±0)% lethality. To test selenium’s potential as an antioxidant in C elegans, we exposed adult animals to increasing concentrations of Na2SeO3 along with the additional presence of 1 or 2 mM H2O2 (Figure 1). With the addition of 1mM Na2SeO3, the lethality rate induced by 1 and 2mM H2O2 decreased to 13.3 (±8.16)% and 76.7 (±18.61)%, respectively. This was a reduction in lethality of 65.2 and 23.3% from the animals exposed to the 1 and 2mM H2O2 alone. These reductions in lethality were observed at concentrations of Na2SeO3, which did not induce significant lethality on their own (no significant difference across all concentrations of Na2SeO3 from 0.001 to 1mM and compared with 0mM by one-way ANOVA). A similar reduction in lethality was observed for animals exposed to 1mM H2O2 and 2mM Na2SeO3, a concentration of selenium that did induce a significant lethality on its own (p < 0.001 when comparing 2mM Na2SeO3 with all concentrations of Na2SeO3 from 0 to 1mM by one-way ANOVA). As the lethality of Na2SeO3 neared the LC50 (3.47mM) calculated for this assay, no further reduction in the peroxide-induced lethality was observed. In contrast, the lethality rate induced by either of the two lowest concentrations of Na2SeO3 tested 0.001 and 0.01mM in combination with 1mM H2O2, increased in comparison with both 1mM H2O2 and the corresponding Na2SeO3 concentration alone. Together, these data suggest that selenium in C elegans has a concentration range in which it may function as an antioxidant protecting against cellular damage, but outside this range it may function to induce free radical formation and cellular damage.
Selenium Induces Oxidative Stress in C elegans
Selenium toxicity is suspected to result from increased levels of oxidative stress (Chen et al., 2007; Spallholz, 1994, 1997), a model which is supported by field studies linking excess environmental selenium to altered glutathione metabolism in aquatic birds (Hoffman, 2002) and by laboratory studies in yeast which showed that both selenomethionine and sodium selenite induced DNA damage through the generation of ROS (Seitomer et al., 2008). Previous studies have shown that in C elegans exposure to other toxic metals or metalloids (e.g., Al, Ar, Pb) can induce oxidative stress (Liao and Yu, 2005; Ye et al., 2008), suggesting that the cytotoxic effects of selenium in C elegans may result from oxidative damage induced through the generation of ROS. These observations lead us to the prediction that ROS levels should increase in C elegans adult animals exposed to excess selenium in their environment. To test this theory, ROS formation was measured in N2 adult animals exposed to Na2SeO3 on agar plates for 2, 4, and 6 h and compared with untreated adults by detecting the oxidation-induced fluorescence emitted by the dye carboxy-H2DCFDA. Levels of the dye were observed to be significantly increased in adult animals treated for 6 h with 5mM Na2SeO3 when compared with the untreated control at the same time point (Figure 2A, and comparing Figure 2C with Figure 2D). No significant difference was observed at the 2- and 4-h time points (compared with their controls at the same time points and across both time points by one-way ANOVA).
Antioxidant treatments have been shown to reduce oxidative damage in C elegans (Kampkötter et al., 2007; Ye et al., 2008). Because excess selenium exposure both increases lethality (Figure 1) and induces ROS formation (Figure 2), it is likely that selenium metabolism results in the formation of pro-oxidants in C elegans. Therefore, we expected that the addition of antioxidants during selenium exposure would diminish the observed ROS formation in adult C elegans animals. Because a recent study showed that depletion of intracellular glutathione resulted in an increased sensitivity to selenite (Wallenberg et al., 2010), we chose to add the cellular antioxidant–reduced glutathione (GSH) along with 5mM Na2SeO3 to our agar plates and exposed the animals for 2, 4, and 6 h to both reagents. No significant difference was detected between the levels of the oxidation-induced fluorescence emitted by the dye carboxy-H2DCFDA for the animals exposed for 6 h to Na2SeO3 and GSH (Figure 2A and 2E) when compared with the control animals at the 6-h time point (Figure 2A and 2C), suggesting that antioxidant treatment resulted in decreased ROS formation. These data together suggest that in C elegans exposures to excess environmental selenium lead to the formation of ROS (as detected by the dye carboxy-H2DCFDA), resulting in the accumulation of oxidative damage sufficient to cause death and that this damage can be prevented by treatment with antioxidants.
GLRX-21 is Required for Protection from Selenium-Induced Lethality
Glutaredoxins are a class of redox proteins that play an essential role in regulating and maintaining the cellular redox homeostasis (Lillig et al., 2008; Shelton et al., 2005). Because of their recently described role in selenium-induced oxidative stress (Lewinska and Bartosz, 2008; Wallenberg et al., 2010), we decided to explore whether any of the four C elegans GLRX proteins, GLRX-5, GLRX-10, GLRX-21 or GLRX-22 mediate the protective effect of GSH against selenium toxicity in the context of a complete multicellular organism. Because the dithiol glutaredoxin, GLRX2 was shown to protect against sodium selenate–induced oxidative damage in the cyanobacteria Synechocystis PCC6803 (Marteyn et al., 2009), we focused on its closest ortholog in C elegans, GLRX-21 (Figure 3A). The C elegans glrx-21 gene is 39% similar to the human glutaredoxin-2 (GLRX2; Figure 3A), which is found in the mitochondria and nucleus of cells (Gladyshev et al., 2001; Lundberg et al., 2001). Worm GLRX-21 is predicted to be located in cytoplasm by the PSORT II algorithm for subcellular localization of proteins (http://psort.ims.u-tokyo.ac.jp/) and is phylogenetically closely related with GLRX-22, another dithiol glutaredoxin of C elegans (Figure 3B). We have found that a strain carrying the mutation tm2921, which deletes the proximal promoter plus the first exon (including the ATG start codon) of the glrx-21 gene, is a null allele as no glrx-21 mRNA is synthesized in the mutant (Figure 3C). The lethality rates measured for populations of adult animals carrying the tm2921 mutation were no different than those of the N2 wild-type animals across all concentrations of Na2Se3 tested (Figure 4A).
Because we had shown that selenium-induced exposures lead to increased ROS formation that was diminished by the addition of reduced GSH (Figure 2), we predicted that the lethality observed in selenium-exposed animals would also be diminished by GSH. Results of a mammalian study in which selenium-fed animals concurrently treated with GSH had reduced mortality rates (Deore et al., 2005) lend additional support to this assumption. Indeed, when adult N2 animals were treated in liquid culture with 5mM Na2SeO3 along with increasing concentrations of reduced GSH, they exhibited a dose-dependent decrease in lethality with the addition of either 1 or 10mM GSH (Figure 4B). However, in contrast to the protective effect observed in the N2 strain with the addition of GSH during selenium exposure, GSH did not rescue the selenium-induced lethality observed for the glrx-21 mutant animals even at 10mM, the highest GSH concentration tested when compared with the selenium only–treated population (Figure 4B).
Selenium Induces Motility Impairment
Movement disorders observed with high-dose selenium consumption by livestock (Panter et al., 1996; Wilson et al., 1983) and humans (Kilness and Hichberg, 1977; Yang et al., 1983) can include mild ataxia, paresis, and paralysis (Koller and Exon, 1986). Because this selenium-induced impaired movement develops progressively with chronic exposures to excess selenium, it may prove to be a more useful measure ultimately than our lethality test for determining the mechanisms of selenium-induced toxicity. Indeed, C elegans movement assays were found successful and in some cases more sensitive than lethality assays to access responses to other toxicological agents (Anderson et al, 2004; Rajini et al., 2008; Sochová et al., 2006). Based on these observations, we predicted that populations of C elegans adult animals exposed to increasing selenium concentrations would exhibit a decrease in their movement prior to death. To test for this, we used a behavioral assay, which measures the effects of selenium exposure on the motile ability (defined in ”Materials and Methods” section) of adult animals placed on agar plates seeded with bacteria and in the presence of various concentrations (0–20mM) of sodium selenite, Na2SeO3. A dose-dependent decrease in motility was observed in populations of animals exposed over time (Figure 5A). In addition, selenomethionine (SeMet) an organic source of selenium, also reduced motility when tested within the same concentration range (Supplementary fig. 2A), but the reduction occurred at lower concentrations and shorter exposure times than that observed for Na2SeO3 making it less useful for long-term studies.
Bacterial Metabolism Does Not Contribute to Selenium-Induced Motility Impairment
The use of feeder organisms (e.g., Escherichia coli) has been identified as a major disadvantage for toxicological testing in C elegans (Sprando et al., 2009). Although animals exposed to selenium for our lethality assay were maintained throughout the assay under starvation conditions, exposures for our behavioral assay included E coli bacteria as a food source. To test whether bacterial metabolism contributed to the observed selenium-induced motility impairment, adult N2 animals were grown with 5mM Na2SeO3 in CeMM, a defined liquid media, which requires no supplementation with a bacterial food source (Szewczyk et al., 2003). Bacterial metabolism of selenium did not appear to be a contributing factor because animals exposed to selenium in defined media still exhibited significantly reduced motility when compared with their untreated controls (p < 0.001 using a two-tailed Student’s t-test; Table 1).
TABLE 1.
The Effect of Bacteria-free Growth Media on the Motile Behavior of Caenorhabditis elegans Adult Animals Exposed to Selenium for 24 h
Yes = nominal concentration of 5mM Na2SeO3.
Averages calculated from four replicate cultures of 100 animals each (n = 400).
GLRX-21 Null Mutation is Hypersensitive to Selenium
Because both the selenium-induced ROS formation (Figure 2A and 2E) and lethality (Figure 4B) were reduced by concurrently growing animals in the presence of GSH, we predicted that the selenium-induced motility impairment would be similarly improved by GSH. This proved to be true because populations of wild-type animals exposed to increasing concentrations (0.7, 1.3, and 2.6mM) of GSH on 5mM Na2SeO3 plates for either 24 or 48 h showed significant improvement in their motility across all the concentrations tested (except 2.6mM at 24 h) when compared with their 0mM controls at the same time point (Figure 5B). Animals exposed for 72 and 96 h to GSH at all concentrations did not show any significant improvements in motility when compared with their selenium-only controls at the same time points (one-way ANOVA; data not shown).
Although the glrx-21 mutation as examined within our lethality assay did not confer greater sensitivity or resistance to selenium, we decided to determine whether it affected the motile behavior on populations of animals grown on agar plates. At a concentration of 5mM Na2SeO3, the glrx-21 mutation tm2921 significantly reduced motility in populations of animals after both 24 and 48 h of exposure to selenium when compared with their wild-type controls at the same time points (Figure 5C). No significant difference in the motile behavior was observed between populations of N2 animals exposed for 24 versus 48 h when compared by two-tailed Student’s t-test. Yet, within the same 24-h time difference, selenium drastically reduced the motility of animals with the glrx-21 mutation from 67.1 (±7.83)% at 24-h exposure to 25.7 (±8.08)% at 48 h (Figure 5C). These data suggest that similar to other studies comparing toxicity assays in C elegans (Sochová et al., 2006), our movement assay proved to be a more sensitive measure of the toxic effects of selenium than did lethality.
Chronic Selenium Exposure Leads to Progression of Motility Impairment
The motility phenotype used to define selenium toxicity above excluded animals that were dead as well as paralyzed but included animals that moved either normally or displayed a backward movement deficit. Collectively these phenotypes could mark a progressive course of injury to animals when they are chronically exposed to excess environmental selenium. In order to test this premise, populations of adult N2 animals were observed at 24-h intervals, and the percentages of the total population exhibiting each of the phenotypes was scored at the various time points (Figure 6A). The distribution of phenotypes was observed to change over time. At the 24-h time point, a significantly larger percentage of animals still exhibited normal movement behavior (p < 0.001 when compared with all other phenotypes at 24 h, one-way ANOVA), whereas the percentages of the other phenotypes were distributed equally (no significant difference for all 24 h phenotypes when compared excluding normal, one-way ANOVA). This distribution of normal moving animals within the exposed populations changed dramatically when the exposure time was increased to 48 h and beyond (p < 0.001 when 24-h normal was compared with normal at all other time points, one-way ANOVA). The percentage of dead animals increased more than threefold from 22.5 (±12.14)% at 24 h to 75.8 (±13.93)% at 48 h, to nearly 100% by 96 h (95.9 ± 4.92%) with accompanying significant drops in the percentages of normal moving, backing-deficient, and paralyzed animals. This process was accelerated in the glrx-21(tm2921) animals, which displayed a distribution of phenotypes at 24 h that was not significantly different from the percentages of phenotypes displayed by N2 at 48 h (compared across all phenotypes, two-way ANOVA; Supplementary fig. 2B). These population studies suggest that continuous exposure to excess selenium leads to a progression of impaired behavior as follows: normal to backing deficient, to paralysis and ultimately death.
This order of progression was confirmed by watching the development of impairment in individual animals. Because the majority of the phenotypic changes were observed to occur within the first 48 h of exposure to excess selenium, individual animals were examined every 6 h for a total of 48 h to more closely examine the phenotypic progression (Figure 6B). When individual animals expressed all behavioral phenotypes, they always progressed from normal to backing deficient to paralysis behavior before dying; although some animals progressed through more than one phenotype within the 6-h period before their next measurement (e.g., Subjects 15-–19 were paralyzed after just 6 h of exposure on selenium).
Acute Exposure to Selenium Alters the Progressive Course of Behavioral Phenotypes
Chronic exposure to excess selenium caused increasing behavioral deficits over time ultimately leading to death, but would an acute exposure be sufficient to cause a similar progression or could a reversal of some or all of the observed deficits occur? To answer this question, animals were first exposed to excess selenium for 24 h after which they were assessed for their behavioral phenotype (Figure 7, “24-h phenotype” denoted in white letters) and then sorted to new selenium-free agar plates. As previously observed with chronic exposure to excess selenium (Figure 6A), populations of normal animals showed a distribution of progressing behaviors after 24 h off of selenium, but a greater percentage of animals did not advance to the more severe phenotypes of paralysis and death (Figure 7). With chronic selenium exposure, approximately 80% of the adult populations were observed to express one of these two phenotypes (paralysis or death) after 48 h of exposure (Figure 6A), whereas only about 25% of each of the populations that were scored as either normal or backing deficient after 24-h exposure were paralyzed or dead after being removed from excess Na2SeO3 for 24 h (Figure 7). In fact, 28.8 (±10.22)%, 65(±5.0)%, and 15(±5.0)% of animals that were normal, backing deficient, or paralyzed, respectively, after 24 h on Na2SeO3 maintained that phenotype 24 h after removal. The observation that 15 (±5.0)% of animals that had originally exhibited a backing deficit after 24-h exposure to Na2SeO3 displayed normal movement 24 h after being removed, suggests that some deficits can be reversed. Individual animals chronically exposed to selenium (Figure 6B) showed no such reversion of phenotypic severity. No reversal to normal or backing-deficient behaviors was observed in animals that were paralyzed after 24 h of exposure; instead, the majority (85 ± 5.0%) had died after 24 h free of Na2SeO3, suggesting that paralysis is a more severe and irreversible phenotype than the backing deficit. Untreated control animals developed none of the behavioral deficits but maintained normal movement throughout this study (Figure 7).
DISCUSSION
The toxicities of a variety of metals and metalloids have been formally assayed in C elegans and include Ag, Al, Ar, Cd, Co, Cr, Cu, Hg, Pb, Ti, Ur, and Zn (Bruinsma et al., 2008; Calafato et al., 2008; Dhawan et al., 2000; Guo et al., 2009; Jiang et al., 2009; Liao and Yu, 2005; Ma et al., 2009; Roh et al., 2009; Wang et al., 2007, 2009; Ye et al., 2008). However, studies on the toxicity of selenium have not been previously reported. Through the results presented here we demonstrate that selenium in the form of Na2SeO3 both prevents and induces oxidative stress in C elegans through a process that involves the GLRX-21 glutaredoxin.
Removal of hydrogen peroxide from cells occurs through the activities of one of three enzymes, glutathione peroxidase, catalase, or peroxiredoxins (Nordberg and Arnér, 2001) but has also been shown to be reduced by a thioredoxin reductase–dependent pathway (Björnstedt et al., 1995). TRXR-1, an ortholog of the human enzymatic antioxidant thioredoxin reductase-1 has been demonstrated to be the only selenoprotein in C elegans (Gladyshev et al., 1999; Taskov et al., 2005). Our observation that at the midrange concentrations (1–2mM) selenium is protective against H2O2-induced toxicity, whereas at the lowest ranges (0.001–0.01mM) selenium is toxic (Figure 1) suggests that in C elegans selenium incorporation into enzymatic antioxidant selenoproteins may be dosage dependent. In previous studies examining the effects of dietary selenium levels on the activity of glutathione peroxidase and thioredoxin reductase, the activity of both enzymes was shown to gradually increase as the concentration of selenium in the feed increased (Hadley and Sunde, 2001; Sunde and Hadley, 2010). Yet, in both cases, selenoprotein activity reached a plateau after which increasing selenium concentration did not increase the activity of the enzymes. In our studies, as selenium concentration neared the Se-LC50 (3.47mM), it ceased to have any measurable antioxidant effect, and increasing lethality was observed within the population as exposure levels increased (Figure 1). With increasing Na2SeO3 concentration, hydrogen selenide (H2Se) produced by the excess selenium would generate ROS causing oxidative stress rather than becoming incorporated into antioxidant selenoproteins (Letavayová, et al., 2006). Our findings of elevated ROS levels in animals exposed to excess selenium (Figure 2) lend support to this theory. Additionally, selenium-dependent oxidation of GSH would in turn further sensitize cells to hydrogen selenide–induced superoxide anions leading to even greater oxidative stress. The dose-dependent ability of glutathione to suppress the ROS formation (Figure 2), lethality (Figure 4B), and motility impairment (Figure 5B) induced by excess selenium is consistent with the predicted depletion of GSH levels by the thiol oxidizing properties of hydrogen selenide and suggests that decreased GSH levels play a key role in the cytotoxic effects of selenium. This is consistent with a recent finding showing that GSH depletion in cells led to the increased sensitivity of cells to selenite (Wallenberg et al., 2010).
The glutaredoxin proteins (GLRXs) are not selenoproteins and thus are not directly affected or regulated by the levels of selenium in cells, but function in the reduction of proteins and low–molecular weight mixed disulfides. Under conditions of oxidative stress, the glutaredoxins can reverse this role and instead catalyze the formation of mixed disulfides, thus preventing further oxidation of thiols such as GSH (Fernandes and Holmgren, 2004; Holmgren, 2000; Lillig et al., 2008). During selenium-induced oxidative stress, the glutaredoxins have been demonstrated to be an essential component in the cellular response of yeast and cyanobacteria (Lewinska and Bartosz, 2008; Marteyn et al., 2009). The acceleration of the timing of motility impairment observed with the loss of the C elegans GLRX-21 protein (Figure 5C and Supplementary fig. 2B) is consistent with a role for glutaredoxin in the cellular responses of C elegans to selenium-induced oxidative stress. In addition, our finding that the loss of functional GLRX-21 protein in worms reversed the suppressive effects of GSH on lethality (Figure 4B), suggests that GLRX-21 is required for this GSH function. GLRX-21 is an ortholog of the human GLRX2 protein (Figure 3A and 3B; Sagemark et al., 2007), which has been shown to reduce mitochondrial apoptotic cell death induced by ROS-producing agents (Holmgren, et al., 2005; Lundberg et al., 2001). Like all GLRX proteins, GLRX2 is maintained in its reduced active form by GSH, which binds to specific sites within the GLRX2 protein that are highly conserved across glutaredoxins (Figure 3A). The data presented here suggest similarly that activation of GLRX-21 by GSH may be required for the reduced lethality observed with increasing GSH concentration in our selenium-treated animals (Figure 4B).
The lethality rate and the LC50 value obtained from our liquid assays provided a useful general measure of the relative toxicity of selenium compared with other metals previously assayed in this model. Yet, as demonstrated in Figure 6A, a 5mM Na2SeO3 exposure of animals on agar plates caused death in only 22.5 (±12.14)% of the animals after 24-h exposure versus 79.2 (±18.01)% of the animals exposed to 5mM Na2SeO3 in our liquid assay with only a 12-h exposure period (Figure 1). This reduction in lethality of animals exposed on agar media allowed the additional observation of a selenium-induced motility phenotype that occurred prior to death (Figure 5). Locomotion in C elegans requires the coordination of three main groups of neurons: sensory neurons, interneurons, and the motor neurons that innervate the body wall muscles (Whittaker and Sternberg 2004). If selenium exposure causes progressive damage to the energy-producing functions of the muscles and/or neurons controlling movement, the resultant reduced energy availability could potentially lead to the observed gradual slowing of movement over time. Yet, our later observations showed that the motility phenotype could be further broken down into two distinct movement defects: impaired backing in response to head tap and paralysis (Figure 6A). In our studies examining single animals chronically exposed to selenium (Figure 6B), the observation that these phenotypes occurred progressively from a period of impaired backing before paralysis and paralysis before dying suggests that there is a stereotypic sequence of cellular damage caused by selenium. Because the ability to coordinate backward movement occurs prior to the more generalized paralysis phenotype, the cells involved in backward movement would appear to be more sensitive to selenium-induced toxicity, whereas those required for forward movement would seem less sensitive. Our observation that a subset of animals that had impaired backing were able to resume normal movement after removal from selenium (Figure 7), suggests that the damage that had been done to those cells was reversible. Thus, the progression of phenotypes could represent the induction of a multistep pathway leading to cellular dysfunction and cell death that could become irreversible by the time enough damage has accumulated. This hypothesis was supported by the fact that paralyzed animals did not recover (Figure 7).
In conclusion, our findings suggest C elegans as an excellent model for elucidating the mechanisms of selenium toxicity. The identification of roles for both glutathione and glutaredoxins in the cellular responses to selenium suggests that many of the processes involved in the selenium-induced toxicity observed in C elegans are similar to those observed within the context of other systems (Izquierdo et al., 2010; Lewinska and Bartosz, 2008; Wallenberg et al., 2010). As a model organism, C elegans has many features that make it an advantageous system for continued analysis on the effects of genes and pharmacological treatments on selenium-induced toxicity including: a short generation time, small nervous system (302 neurons), and conserved cellular signaling pathways (Riddle et al., 1997). These distinctive advantages of C elegans have proved very useful to investigations examining the impact of environmental toxicants (Leung et al., 2008; Peterson et al., 2008) and to models of medically relevant disease processes as diverse as Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, apoptosis, and stroke (Faber et al., 2002; Horvitz et al., 1994; Levitan and Greenwald, 1998; Nass et al., 2008; Scott et al., 2002; Westlund et al., 1999). The movement assay described herein should provide a sensitive tool for further genetic and molecular analysis of selenium-induced toxicity.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported by a Career Development Award (M.E.) provided through the Office of Research and Development, Department of Veterans Affairs, the National Institutes of Environmental Health Sciences (R21-ES012305 to A.E. and M.E.) and Arthritis and Musculoskeletal and Skin Diseases (R01-AR054342 to N.J.S.), the Instituto de Salud Carlos III (Projects PI050065 and PI080557, co-financed with the Fondo Social Europeo, FEDER) and Junta de Andalucía (Projects P07-CVI-02697 and P08-CVI-03629), Spain (A.M.-V.) and a predoctoral fellowship from CONACYT, Mexico (B.C.-V.).
Supplementary Material
Acknowledgments
Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR) and by the Japanese C elegans Gene Knockout Consortia funded by the National Bioresource Project and led by S. Mitani at Tokyo Women's Medical University School of Medicine. We would like to thank Dr Philip Atherton for GraphPad tutelage, and Francisco José Naranjo for his excellent technical assistance with RT-PCR.
References
- Ammar EM, Couri D. Acute toxicity of sodium selenite and selenomethionine in mice after icv or iv administration. Neurotoxicology. 1981;2:383–386. [PubMed] [Google Scholar]
- Anderson GL, Cole RD, Williams PL. Assessing behavioral toxicity with Caenorhabditis elegans. Environ. Toxicol. Chem. 2004;23:1235–1240. doi: 10.1897/03-264. [DOI] [PubMed] [Google Scholar]
- Batist G, Katki AG, Klecker RW, Jr, Myers CE. Selenium-induced cytotoxicity of human leukemia cells: interaction with reduced glutathione. Cancer Res. 1986;46:5482–5485. [PubMed] [Google Scholar]
- Battin EE, Brumaghim JL. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009;55:1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- Björnstedt M, Hamberg M, Kumar S, Xue J, Holmgren A. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J. Biol. Chem. 1995;270:11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- Boosalis MG. The role of selenium in chronic disease. Nutr. Clin. Pract. 2008;23:152–160. doi: 10.1177/0884533608314532. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma JJ, Schneide DL, Davis DE, Kornfeld K. Identification of mutations in Caenorhabditis elegans that cause resistance to high levels of dietary zinc and analysis using a genomewide map of single nucleotide polymorphisms scored by pyrosequencing. Genetics. 2008;179:811–828. doi: 10.1534/genetics.107.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafato S, Swain S, Hughes S, Kille P, Stürzenbaum AR. Knock down of Caenorhabditis elegans cutc-1 exacerbates the sensitivity toward high levels of copper. Toxicol. Sci. 2008;106:384–391. doi: 10.1093/toxsci/kfn180. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Boylan LM, Wu CK, Spallholz JE. Oxidation of glutathione and superoxide generation by inorganic and organic selenium compounds. Biofactors. 2007;31:55–66. doi: 10.1002/biof.5520310106. [DOI] [PubMed] [Google Scholar]
- Deore MD, Srivastava AK, Sharma SK. Effect of reduced glutathione treatment on selenosis, blood selenium concentration and glutathione peroxidase activity after repeated short-term selenium exposure in buffalo calves. Toxicology. 2005;213:169–174. doi: 10.1016/j.tox.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Dhawan R, Dusenbery DB, Williams PL. A comparison of metal-induced lethality and behavioral responses in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2000;19:3061–3067. [Google Scholar]
- Estevez M, Estevez AO, Cowie RH, Gardner KL. The voltage-gated calcium channel UNC-2 is involved in stress-mediated regulation of tryptophan hydroxylase. J. Neurochem. 2004;88:102–113. doi: 10.1046/j.1471-4159.2003.02140.x. [DOI] [PubMed] [Google Scholar]
- Facompre N, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: implications for cancer survivors. Cancer Res. 2009;69:2699–2703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber PW, Voisine C, King DC, Bates EA, Hart AC. Glutamine/proline-rich PQE-1 proteins protect Caenorhabditis elegans neurons from huntingtin polyglutamine neurotoxicity. Proc. Natl Acad. Sci. U S A. 2002;99:17131–17136. doi: 10.1073/pnas.262544899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am. J. Clin. Nutr. 1993;57(Suppl.):259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Di Simplicio P. Protein glutathionylation. In: Jacob C, Winiyard PG, editors. Redox Signaling and Regulation in Biology and Medicine. Weinheim, Germany: Wiley-VCH; 2009. pp. 123–141. [Google Scholar]
- Gladyshev VN, Krause M, Xu XM, Korotkov KV, Kryukov GV, Sun QA, Lee BJ, Wootton JC, Hatfield DL. Selenocysteine-containing thioredoxin reductase in C. elegans. Biochem. Biophys. Res. Commun. 1999;259:244–249. doi: 10.1006/bbrc.1999.0765. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J. Biol. Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- Guo Y, Yang Y, Wang D. Induction of reproductive deficits in nematode Caenorhabditis elegans exposed to metals at different developmental stages. Reprod. Toxicol. 2009;28:90–95. doi: 10.1016/j.reprotox.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Hadley KB, Sunde RA. Selenium regulation of thioredoxin reductase activity and mRNA levels in rat liver. J. Nutr. Biochem. 2001;12:693–702. doi: 10.1016/s0955-2863(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ. Role of selenium toxicity and oxidative stress in aquatic birds. Aquat. Toxicol. 2002;57:11–26. doi: 10.1016/s0166-445x(01)00263-6. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Johansson C, Berndt C, Lönn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem. Soc. Trans. 2005;33(Pt 6):1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Shaham S, Hengartner MO. The genetics of programmed cell death in the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1994;59:377–385. doi: 10.1101/sqb.1994.059.01.042. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Casas C, Herrero E. Selenite-induced cell death in Saccharomyces cerevisiae protective role of glutaredoxins. Microbiology. 2010 doi: 10.1099/mic.0.039719-0. 156(Pt. 9), 2608–2620. [DOI] [PubMed] [Google Scholar]
- Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:718–726. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- Jiang GC, Hughes S, Stürzenbaum SR, Evje L, Syversen T, Aschner M. Caenorhabditis elegans metallothioneins protect against toxicity induced by depleted uranium. Toxicol Sci. 2009;111:345–354. doi: 10.1093/toxsci/kfp161. [DOI] [PubMed] [Google Scholar]
- Kampkötter A, Nkwonkam CG, Zurawski RF, Timpel C, Chovolou Y, Wätjen W, Kahl R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology. 2007;234:113–123. doi: 10.1016/j.tox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Kilness AW, Hichberg FH. Amyotrophic lateral sclerosis in a high selenium environment. JAMA. 1977;237:2843–2844. [PubMed] [Google Scholar]
- Kim YY, Mahan DC. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 2001;79:942–948. doi: 10.2527/2001.794942x. [DOI] [PubMed] [Google Scholar]
- Koller LD, Exon JH. The two faces of selenium-deficiency and toxicity–are similar in animals and man. Can. J. Vet. Res. 1986;50:297–306. [PMC free article] [PubMed] [Google Scholar]
- Kumsta C, Thamsen M, Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxid. Redox Signal. 2010 doi: 10.1089/ars.2010.3203. doi:10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemly AD. Environmental implications of excessive selenium: a review. Biomed. Environ. Sci. 1997;10:415–435. [PubMed] [Google Scholar]
- Lenz M, Lens PN. The essential toxin: the changing perception of selenium in environmental sciences. Sci. Total Environ. 2009;407:3620–3633. doi: 10.1016/j.scitotenv.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Letavayová L, Vlcková V, Brozmanová J. Selenium: from cancer prevention to DNA damage. Toxicology. 2006;227:1–14. doi: 10.1016/j.tox.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development. 1998;125:3599–3606. doi: 10.1242/dev.125.18.3599. [DOI] [PubMed] [Google Scholar]
- Lewinska A, Bartosz G. A role for yeast glutaredoxin genes in selenite-mediated oxidative stress. Fungal Genet Biol. 2008;45:1182–1187. doi: 10.1016/j.fgb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Liao VH, Yu CW. Caenorhabditis elegans gcs-1 confers resistance to arsenic-induced oxidative stress. Biometals. 2005;18:519–528. doi: 10.1007/s10534-005-2996-3. [DOI] [PubMed] [Google Scholar]
- Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim. Biophys. Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Lu J, Berndt C, Holmgren A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim. Biophys. Acta. 2009;1790:1513–1519. doi: 10.1016/j.bbagen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Luke CJ, Pak SC, Askew YS, Naviglia TL, Askew DJ, Nobar SM, Vetica AC, Long OS, Watkins SC, Stolz DB, et al. An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell. 2007;130:1108–1119. doi: 10.1016/j.cell.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J. Biol. Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- Ma H, Glenn TC, Jagoe CH, Jones KL, Williams PL. A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ. Toxicol. Chem. 2009;28:1311–1318. doi: 10.1897/08-496.1. [DOI] [PubMed] [Google Scholar]
- MacDonald DW, Christian RG, Strausz KI, Roff J. Acute selenium toxicity in neonatal calves. Can. Vet. J. 1981;22:79–81. [PMC free article] [PubMed] [Google Scholar]
- Marteyn B, Domain F, Legrain P, Chauvat F, Cassier-Chauvat C. The thioredoxin reductase-glutaredoxins-ferredoxin crossroad pathway for selenate tolerance in Synechocystis PCC6803. Mol. Microbiol. 2009;71:520–532. doi: 10.1111/j.1365-2958.2008.06550.x. [DOI] [PubMed] [Google Scholar]
- Misra S, Niyogi S. Selenite causes cytotoxicity in rainbow trout (Oncorhynchus mykiss) hepatocytes by inducing oxidative stress. Toxicol. In Vitro. 2009;23:1249–1258. doi: 10.1016/j.tiv.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Nass R, Merchant KM, Ryan T. Caenohabditis elegans in Parkinson's disease drug discovery: addressing an unmet medical need. Mol. Interv. 2008;8:284–293. doi: 10.1124/mi.8.6.6. [DOI] [PubMed] [Google Scholar]
- Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci. Total Environ. 2008;400:115–141. doi: 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arnér ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Palmer MA, Bernhardt ES, Schlesinger WH, Eshleman KN, Foufoula-Georgiou E, Hendryx MS, Lemly AD, Likens GE, Loucks OL, Power ME, et al. Science and regulation. Mountaintop mining consequences. Science. 2010;327:148–149. doi: 10.1126/science.1180543. [DOI] [PubMed] [Google Scholar]
- Panter KE, Hartley WJ, James LF, Mayland HF, Stegelmeier BL, Kechele PO. Comparative toxicity of selenium from seleno-DL-methionine, sodium selenate, and Astragalus bisculcatusin pigs. Fund. Appl. Toxicol. 1996;32:217–223. [PubMed] [Google Scholar]
- Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajini PS, Melstrom P, Williams PL. A comparative study on the relationship between various toxicological endpoints in Caenorhabditis elegans exposed to organophosphorus insecticides. J. Toxicol. Environ. Health A. 2008;71(15):1043–1050. doi: 10.1080/15287390801989002. [DOI] [PubMed] [Google Scholar]
- Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol. Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Blumnethal T, Meyer B, Priess J. C. elegans II. New York, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ. Sci. Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Sagemark J, Elgán TH, Bürglin TR, Johansson C, Holmgren A, Berndt KD. Redox properties and evolution of human glutaredoxins. Proteins. 2007;68:879–892. doi: 10.1002/prot.21416. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Scott BA, Avidan MS, Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002;296:2388–2391. doi: 10.1126/science.1072302. [DOI] [PubMed] [Google Scholar]
- See KA, Lavercombe PS, Dillon J, Ginsberg R. Accidental death from acute selenium poisoning. Med. J. Aust. 2006;185:388–389. doi: 10.5694/j.1326-5377.2006.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Seitomer E, Balar B, He D, Copeland PR, Kinzy TG. Analysis of Saccharomyces cerevisiae null allele strains identifies a larger role for DNA damage versus oxidative stress pathways in growth inhibition by selenium. Mol. Nutr. Food Res. 2008;52:1305–1315. doi: 10.1002/mnfr.200700347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- Sochová I, Hofman J, Holoubek I. Using nematodes in soil ecotoxicology. Environ. Int. 2006;32:374–383. doi: 10.1016/j.envint.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994;17:54–64. doi: 10.1016/0891-5849(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Spallholz JE. Free radical generation by selenium compounds and their pro-oxidant toxicity. Biomed. Environ. Sci. 1997;10:260–270. [PubMed] [Google Scholar]
- Spallholz JE, Hoffman DJ. Selenium toxicity: cause and effects in aquatic birds. Aquat. Toxicol. 2002;57:27–37. doi: 10.1016/s0166-445x(01)00268-5. [DOI] [PubMed] [Google Scholar]
- Sprando RL, Olejnik N, Cinar HN, Ferguson M. A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a complex object parametric analyzer and sorter, and axenic liquid media. Food Chem. Toxicol. 2009;47:722–728. doi: 10.1016/j.fct.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C. elegans. A Practical Approach. New York, NY: Oxford University Press; 1999. pp. 51–67. [Google Scholar]
- Sunde RA, Hadley KB. Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp. Biol. Med. (Maywood). 2010;235:23–31. doi: 10.1258/ebm.2009.009262. [DOI] [PubMed] [Google Scholar]
- Szewczyk NJ, Kozak E, Conley CA. Chemically defined medium and Caenorhabditis elegans. BMC Biotechnol. 2003;3:19. doi: 10.1186/1472-6750-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskov K, Chapple C, Kryukov GV, Castellano S, Lobanov AV, Korotkov KV, Guigó R, Gladyshev VN. Nematode selenoproteome: the use of the selenocysteine insertion system to decode one codon in an animal genome? Nucleic Acids Res. 2005;33:2227–2238. doi: 10.1093/nar/gki507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary AK, Stegelmeier BL, Panter KE, James LF, Hall JO. Comparative toxicosis of sodium selenite and selenomethionine in lambs. J. Vet. Diagn. Invest. 2006;18:61–70. doi: 10.1177/104063870601800108. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Guidetti D, Pinotti M, Rovesti S, Merlin M, Vescov L, Bergomi M, Vivoli G. Amyotrophic lateral sclerosis after long-term exposure to drinking water with high selenium content. Epidemiology. 1996;7:529–532. [PubMed] [Google Scholar]
- Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G. Adverse health effects of selenium in humans. Rev. Environ. Health. 2001;16:233–251. doi: 10.1515/reveh.2001.16.4.233. [DOI] [PubMed] [Google Scholar]
- Wallenberg M, Olm E, Hebert C, Björnstedt M, Fernandes AP. Selenium compounds are substrates for glutaredoxins: a novel pathway for selenium metabolism and a potential mechanism for selenium mediated cytotoxicity. Biochem. J. 2010;429:85–93. doi: 10.1042/BJ20100368. [DOI] [PubMed] [Google Scholar]
- Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009;157:1171–1177. doi: 10.1016/j.envpol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xie W, Wang D. Transferable properties of multi-biological toxicity caused by cobalt exposure in Caenorhabditis elegans. Environ. Toxicol. Chem. 2007;26:2405–2412. doi: 10.1897/06-646R1.1. [DOI] [PubMed] [Google Scholar]
- Westlund B, Parry D, Clover R, Basson M, Johnson CD. Reverse genetic analysis of Caenorhabditis elegans presenilins reveals redundant but unequal roles for sel-12 and hop-1 in Notch-pathway signaling. Proc. Natl Acad. Sci. U S A. 1999;96:2497–2502. doi: 10.1073/pnas.96.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker AJ, Sternberg PW. Sensory processing by neural circuits in Caenorhabditis elegans. Curr. Opin. Neurobiol. 2004;14:450–456. doi: 10.1016/j.conb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Wilson TM, Scholz RW, Drake TR. Selenium toxicity and porcine focal symmetrical poliomyelomalacia: description of a field outbreak and experimental reproduction. Can. J. Comp. Med. 1983;47:412–421. [PMC free article] [PubMed] [Google Scholar]
- Yamniuk AP, Ishida H, Lippert D, Vogel HJ. Thermodynamic effects of noncoded and coded methionine substitutions in calmodulin. Biophys. J. 2009;96:1495–1507. doi: 10.1016/j.bpj.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GQ, Wang SZ, Zhou RH, Sun SZ. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983;37:872–881. doi: 10.1093/ajcn/37.5.872. [DOI] [PubMed] [Google Scholar]
- Ye H, Ye B, Wang D. Trace administration of vitamin E can retrieve and prevent UV-irradiation- and metal exposure-induced memory deficits in nematode Caenorhabditis elegans. Neurobiol. Learn. Mem. 2008;90:10–18. doi: 10.1016/j.nlm.2007.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.