Abstract
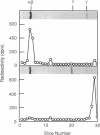
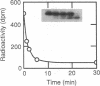
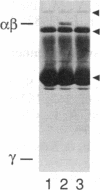
Retinal rod cGMP phosphodiesterase (3',5'-cyclic-GMP phosphodiesterase; EC 3.1.4.35; PDE), a key regulatory enzyme involved in visual excitation, is one of several outer segment membrane proteins that are carboxyl methylated in the presence of the methyl donor S-adenosyl-L-[3H-methyl]methionine. By chromatographic analyses of the 3H-methyl amino acid generated by exhaustive proteolysis of purified PDE, followed by performic acid oxidation of the digest, we have shown that this modification occurs at a C-terminal cysteine residue of the alpha subunit of this enzyme. When PDE is subjected to limited proteolysis with trypsin, a 3H-methylated fragment of 1000 daltons or less is rapidly removed prior to the degradation of its inhibitory gamma subunit. This small fragment remains membrane bound, whereas the bulk of the enzyme is released, indicating that a domain responsible for anchoring PDE to the membrane is located near the C terminus. Based on the C-terminal amino acid sequence of Cys-Cys-Val-Gln predicted from the alpha cDNA sequence, we conclude that PDE undergoes posttranslational modifications, including the proteolytic removal of two or three terminal amino acids, and methyl esterification of the alpha-carboxyl group of the terminal cysteine residue. We speculate that the sulfhydryl group of the methylated cysteine is also lipidated to mediate membrane binding. These modifications may play an important role in delivering the nascent PDE chains to the membrane and in correctly positioning the PDE molecule in the rod disks for phototransduction.
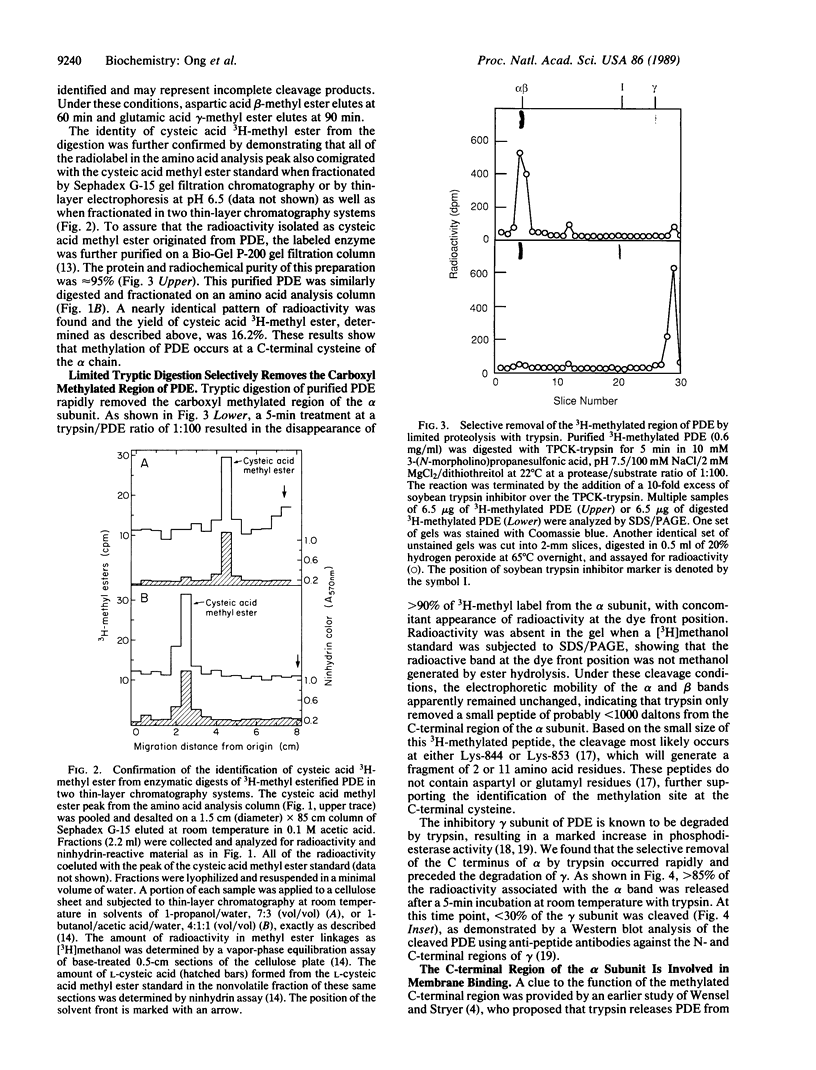
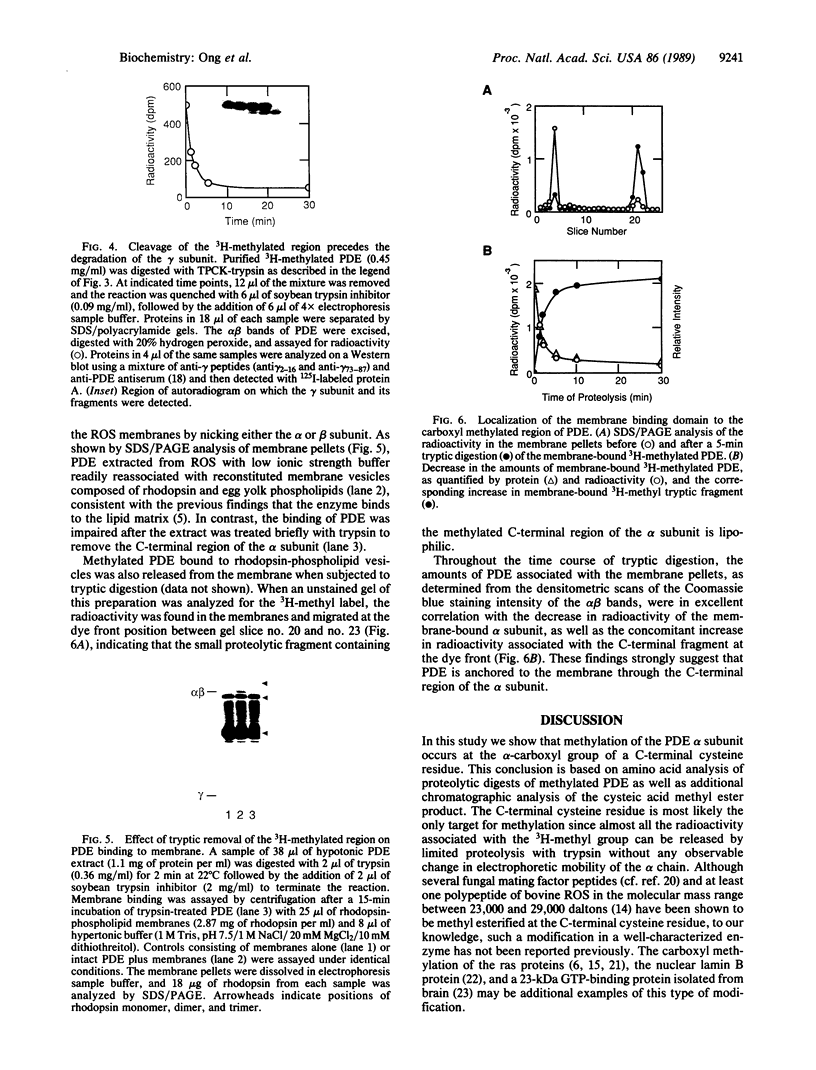
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Olson J. F., Koshland D. E., Jr Cell cycle-dependent methyl esterification of lamin B. J Biol Chem. 1987 Mar 25;262(9):4303–4309. [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Stimmel J. B., Clarke S., Stock J., Broach J. R. RAS2 protein of Saccharomyces cerevisiae is methyl-esterified at its carboxyl terminus. J Biol Chem. 1989 Jul 15;264(20):11865–11873. [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Fung B. K., Griswold-Prenner I. G protein-effector coupling: binding of rod phosphodiesterase inhibitory subunit to transducin. Biochemistry. 1989 Apr 18;28(8):3133–3137. doi: 10.1021/bi00434a003. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Miller J. L., Litman B. J., Dratz E. A. Binding and activation of rod outer segment phosphodiesterase and guanosine triphosphate binding protein by disc membranes: influence of reassociation method and divalent cations. Biochim Biophys Acta. 1987 Mar 26;898(1):81–89. doi: 10.1016/0005-2736(87)90111-8. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Murray E. D., Jr, Clarke S. Metabolism of a synthetic L-isoaspartyl-containing hexapeptide in erythrocyte extracts. Enzymatic methyl esterification is followed by nonenzymatic succinimide formation. J Biol Chem. 1986 Jan 5;261(1):306–312. [PubMed] [Google Scholar]
- Ota I. M., Clarke S. Enzymatic methylation of 23-29-kDa bovine retinal rod outer segment membrane proteins. Evidence for methyl ester formation at carboxyl-terminal cysteinyl residues. J Biol Chem. 1989 Aug 5;264(22):12879–12884. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Swanson R. J., Applebury M. L. Methylation of proteins in photoreceptor rod outer segments. J Biol Chem. 1983 Sep 10;258(17):10599–10605. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyminski P. N., O'Brien D. F. Rod outer segment phosphodiesterase binding and activation in reconstituted membranes. Biochemistry. 1984 Aug 14;23(17):3986–3993. doi: 10.1021/bi00312a028. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Norris K., Papageorge A. G., Hubbert N. L., Lowy D. R. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984 Nov;3(11):2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]