Abstract
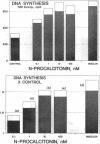
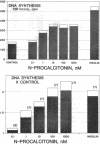
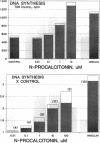
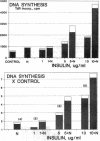
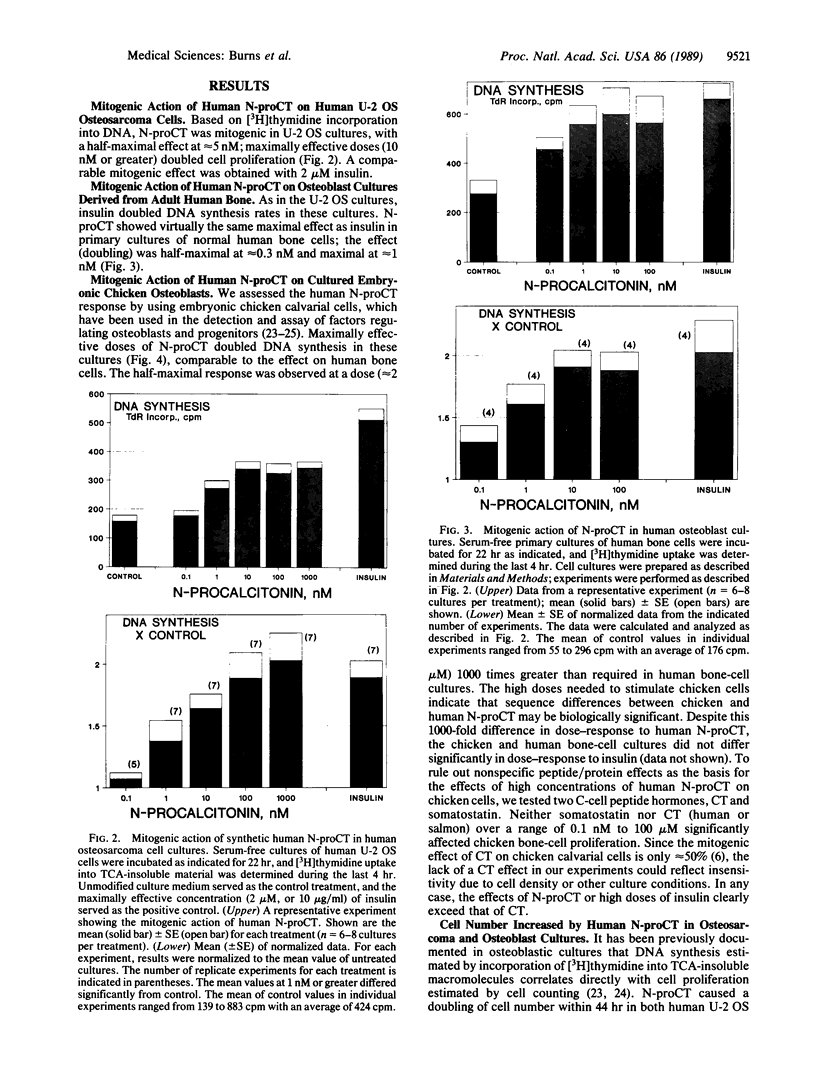
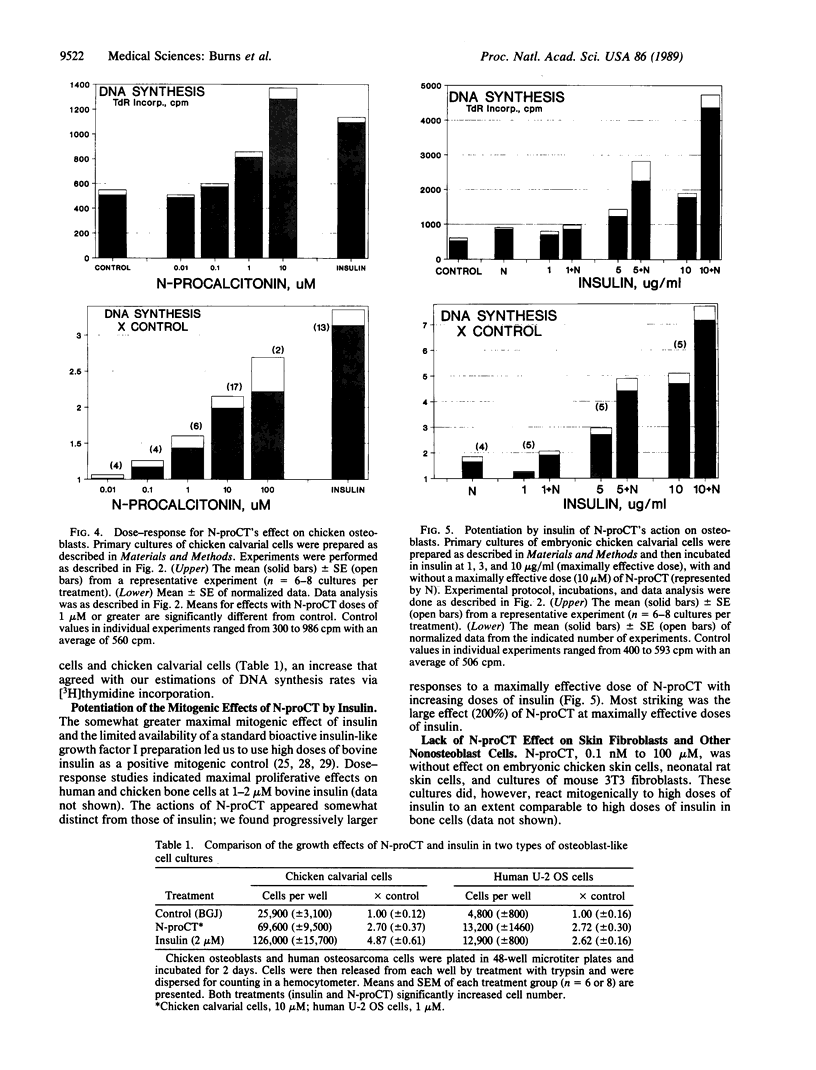
The parafollicular-cell (C-cell) hormone calcitonin (CT) can preserve or even augment skeletal mass by inhibiting osteoclast-mediated bone resorption. The possibility of an additional anabolic skeletal influence has also been raised: C cells might, via CT or other secretory products, affect osteoblast-mediated bone formation. The 57-residue amino-terminal procalcitonin cleavage peptide, N-proCT, has recently been identified in human and rat C cells, where it is made and secreted in equimolar amounts with CT. The coelaboration of N-proCT and CT and N-proCT's sequence conservation during evolution prompted us to investigate the potential skeletal bioactivity of N-proCT. We found that synthetic human N-proCT, at nanomolar concentrations, stimulated proliferation of normal and neoplastic human osteoblasts. At maximally effective doses, human N-proCT caused more than a 100% increase above the control rate of DNA synthesis, an effect comparable to the maximal growth effect of insulin, a potent mitogen for osteoblasts. Human N-proCT exerted a similar maximal mitogenic effect in chicken osteoblast cultures but at 1000-fold greater concentrations than in human bone-cell cultures. The bone-cell action of N-proCT was potentiated with insulin with a greater than 200% increase in DNA synthesis at high insulin concentrations. In sharp contrast to these findings for N-proCT, the other bioactive C-cell peptides, CT and somatostatin, showed no mitogenic effects in human or chicken osteoblast cultures. Our results indicate that the action of N-proCT on cultured bone cells is separate from and potentiated by insulin, a known growth factor. Unlike insulin and related growth factors such as insulin-like growth factor I, N-proCT is not mitogenic in skin fibroblast cultures. We propose that N-proCT is a C-cell hormone that promotes bone formation via stimulatory actions on osteoblasts and preosteoblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Young E., Walker J. M., Watson S. J. The many possible roles of opioids and related peptides in stress-induced analgesia. Ann N Y Acad Sci. 1986;467:140–153. doi: 10.1111/j.1749-6632.1986.tb14625.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum R. S., O'Neil J. A., Muszynski M., Aron D. C., Roos B. A. A non-calcitonin secretory peptide derived from preprocalcitonin. J Biol Chem. 1982 Jan 10;257(1):241–244. [PubMed] [Google Scholar]
- Breimer L. H., MacIntyre I., Zaidi M. Peptides from the calcitonin genes: molecular genetics, structure and function. Biochem J. 1988 Oct 15;255(2):377–390. doi: 10.1042/bj2550377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. M., Birnbaum R. S., Roos B. A. A neuroendocrine peptide derived from the amino-terminal half of rat procalcitonin. Mol Endocrinol. 1989 Jan;3(1):140–147. doi: 10.1210/mend-3-1-140. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Grimelius L., Thim L. Structural characterization of a high-molecular-mass form of calcitonin [procalcitonin-(60-116)-peptide] and its corresponding N-terminal flanking peptide [procalcitonin-(1-57)-peptide] in a human medullary thyroid carcinoma. Biochem J. 1988 Nov 15;256(1):245–250. doi: 10.1042/bj2560245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover C. A., Hintz R. L., Rosenfeld R. G. Comparative effects of somatomedin C and insulin on the metabolism and growth of cultured human fibroblasts. J Cell Physiol. 1985 Jan;122(1):133–141. doi: 10.1002/jcp.1041220120. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Krook L., Mayer G. P. Plasma calcitonin in the bovine species. Proc Soc Exp Biol Med. 1979 Oct;162(1):150–151. doi: 10.3181/00379727-162-40635. [DOI] [PubMed] [Google Scholar]
- Drivdahl R. H., Howard G. A., Baylink D. J. Extracts of bone contain a potent regulator of bone formation. Biochim Biophys Acta. 1982 Jan 12;714(1):26–33. doi: 10.1016/0304-4165(82)90123-4. [DOI] [PubMed] [Google Scholar]
- Eilon G., Perkins J., Viola M. V. Characteristics of a calcitonin-responsive cell line derived from a human osteosarcoma. Cancer Res. 1983 Aug;43(8):3763–3769. [PubMed] [Google Scholar]
- Ernst M., Froesch E. R. Growth hormone dependent stimulation of osteoblast-like cells in serum-free cultures via local synthesis of insulin-like growth factor I. Biochem Biophys Res Commun. 1988 Feb 29;151(1):142–147. doi: 10.1016/0006-291x(88)90570-0. [DOI] [PubMed] [Google Scholar]
- Ernst M., Schmid C., Froesch E. R. Enhanced osteoblast proliferation and collagen gene expression by estradiol. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2307–2310. doi: 10.1073/pnas.85.7.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivariz F. E., Carino M., Lowry P. J., Jackson S. Further evidence that N-terminal pro-opiomelanocortin peptides are involved in adrenal mitogenesis. J Endocrinol. 1988 Feb;116(2):201–206. doi: 10.1677/joe.0.1160201. [DOI] [PubMed] [Google Scholar]
- Farley J. R., Tarbaux N. M., Hall S. L., Linkhart T. A., Baylink D. J. The anti-bone-resorptive agent calcitonin also acts in vitro to directly increase bone formation and bone cell proliferation. Endocrinology. 1988 Jul;123(1):159–167. doi: 10.1210/endo-123-1-159. [DOI] [PubMed] [Google Scholar]
- Ferrier J., Ward-Kesthely A., Heersche J. N., Aubin J. E. Membrane potential changes, cAMP stimulation and contraction in osteoblast-like UMR 106 cells in response to calcitonin and parathyroid hormone. Bone Miner. 1988 Jun;4(2):133–145. [PubMed] [Google Scholar]
- Gkonos P. J., Born W., Jones B. N., Petermann J. B., Keutmann H. T., Birnbaum R. S., Fischer J. A., Roos B. A. Biosynthesis of calcitonin gene-related peptide and calcitonin by a human medullary thyroid carcinoma cell line. J Biol Chem. 1986 Nov 5;261(31):14386–14391. [PubMed] [Google Scholar]
- Hickman J., McElduff A. Insulin promotes growth of the cultured rat osteosarcoma cell line UMR-106-01: an osteoblast-like cell. Endocrinology. 1989 Feb;124(2):701–706. doi: 10.1210/endo-124-2-701. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Turner R. T., Sherrard D. J., Baylink D. J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981 Aug 10;256(15):7738–7740. [PubMed] [Google Scholar]
- Höppener J. W., Steenbergh P. H., Slebos R. J., de Pagter-Holthuizen P., Roos B. A., Jansen M., Van den Brande J. L., Sussenbach J. S., Jansz H. S., Lips C. J. Expression of insulin-like growth factor-I and -II genes in rat medullary thyroid carcinoma. FEBS Lett. 1987 May 4;215(1):122–126. doi: 10.1016/0014-5793(87)80125-4. [DOI] [PubMed] [Google Scholar]
- Kalu D. N., Cockerham R., Yu B. P., Roos B. A. Lifelong dietary modulation of calcitonin levels in rats. Endocrinology. 1983 Dec;113(6):2010–2016. doi: 10.1210/endo-113-6-2010. [DOI] [PubMed] [Google Scholar]
- Krook L., Lutwak L., McEntee K. Dietary calcium, ultimobranchial tumors and osteopetrosis in the bull. Syndrome of calcitonin excess? Am J Clin Nutr. 1969 Feb;22(2):115–118. doi: 10.1093/ajcn/22.2.115. [DOI] [PubMed] [Google Scholar]
- Lasmoles F., Jullienne A., Day F., Minvielle S., Milhaud G., Moukhtar M. S. Elucidation of the nucleotide sequence of chicken calcitonin mRNA: direct evidence for the expression of a lower vertebrate calcitonin-like gene in man and rat. EMBO J. 1985 Oct;4(10):2603–2607. doi: 10.1002/j.1460-2075.1985.tb03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre I., Hillyard C. J., Murphy P. K., Reynolds J. J., Das R. E., Craig R. K. A second plasma calcium-lowering peptide from the human calcitonin precursor. Nature. 1982 Dec 2;300(5891):460–462. doi: 10.1038/300460a0. [DOI] [PubMed] [Google Scholar]
- Pfeifle B., Boeder H., Ditschuneit H. Interaction of receptors for insulin-like growth factor I, platelet-derived growth factor, and fibroblast growth factor in rat aortic cells. Endocrinology. 1987 Jun;120(6):2251–2258. doi: 10.1210/endo-120-6-2251. [DOI] [PubMed] [Google Scholar]
- Puzas J. E., Drivdahl R. H., Howard G. A., Baylink D. J. Endogenous inhibitor of bone cell proliferation. Proc Soc Exp Biol Med. 1981 Jan;166(1):113–122. doi: 10.3181/00379727-166-41032. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988 Mar 31;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Roos B. A., Fischer J. A., Pignat W., Alander C. B., Raisz L. G. Evaluation of the in vivo and in vitro calcium-regulating actions of noncalcitonin peptides produced via calcitonin gene expression. Endocrinology. 1986 Jan;118(1):46–51. doi: 10.1210/endo-118-1-46. [DOI] [PubMed] [Google Scholar]
- Roos B. A., Huber M. B., Birnbaum R. S., Aron D. C., Lindall A. W., Lips K., Baylin S. B. Medullary thyroid carcinomas secrete a noncalcitonin peptide corresponding to the carboxyl-terminal region of preprocalcitonin. J Clin Endocrinol Metab. 1983 Apr;56(4):802–807. doi: 10.1210/jcem-56-4-802. [DOI] [PubMed] [Google Scholar]
- Roos B. A., Okano K., Deftos L. J. Evidence for a pro-calcitonin. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1134–1140. doi: 10.1016/0006-291x(74)90430-6. [DOI] [PubMed] [Google Scholar]
- Shamonki I. M., Frumar A. M., Tataryn I. V., Meldrum D. R., Davidson B. H., Parthemore J. G., Judd H. L., Deftos L. J. Age-related changes of calcitonin secretion in females. J Clin Endocrinol Metab. 1980 Mar;50(3):437–439. doi: 10.1210/jcem-50-3-437. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Chambers T. J., Bevis P. J., Beacham J. L., Gaines Das R. E., MacIntyre I. Effects of peptides from the calcitonin genes on bone and bone cells. Q J Exp Physiol. 1988 Jul;73(4):471–485. doi: 10.1113/expphysiol.1988.sp003168. [DOI] [PubMed] [Google Scholar]