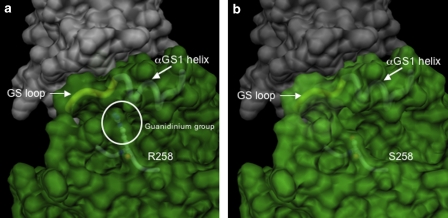
Figure 3.
Representation of the molecular surface of the ACVR1: FKPB12 complex in the proximity of the mutated site. (a) Wild-type ACVR1. On account of the strong similarity between the catalytic domain of TGFBR1 and ACVR1, the quaternary arrangement of the ACVR1: FKPB12 complex is predicted to be the same as that observed in the crystal structure of the TGFBR1: FKPB12 complex.22 The surfaces of the two proteins were calculated independently with the MSMS program suite32, 33 with a conventional probe radius of 1.4 Å. The residue, R258, is represented in ball-and-stick, visible under the semitransparent surface, together with the main chain in its close proximity. The main chain of the αGS1 helix and the GS loop are schematically represented and indicated, the circle encloses the guanidium group of the R258 residue. (b) R258S mutation. The shorter serine side chain is no longer in interaction with the GS region.