Abstract
Thiopurine S-methyltransferase (TPMT) is the rate-limiting step in the conversion of thiopurine drugs including azathioprine (AZA) to inactive metabolites. Heritable deficiency of TPMT activity increases risk for adverse events, most notably, myelosuppression leading to leukopenia and neutropenic sepsis. The reported European Commission study was undertaken to identify current evidence for the clinical utility of testing for TPMT status and extent of uptake, by either genotyping or phenotyping, in the clinical setting. Data presented here for the UK and Spain indicate that there has been a considerable increase in the uptake of TPMT testing in recent years. There are some data that support routine TPMT testing before AZA prescribing for reducing AZA-related adverse events. Key data include evidence in favor of TPMT testing in addition to the current practice of routine monitoring for reducing the number of AZA-related episodes of myelosuppression, averting deaths from neutropenic sepsis and improving health-related quality of life. Further data are needed for determining the cost-effectiveness of routine TPMT testing.
Keywords: pharmacogenetics, mercaptopurines, azathioprine, neutropenia, genetic testing, thiopurine S-methyltransferase
Introduction
Azathioprine (AZA) is among the most commonly used medicines for immunosuppression following organ transplantation, and for the chronic treatment of autoimmune diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD).1, 2, 3, 4 It is also used in the treatment of multiple sclerosis.5 AZA is also widely used in dermatology for treating immunobullous diseases, generalized eczematous disorders, and photodermatoses.6 AZA is a generic drug, first introduced into clinical practice for kidney transplant patients in the UK during the early 1960s. It is produced by a number of generic manufacturers and marketed under several brand names (Imuran by GlaxoSmithKline in Canada, Australia, and UK; Azasan by Salix in the USA).
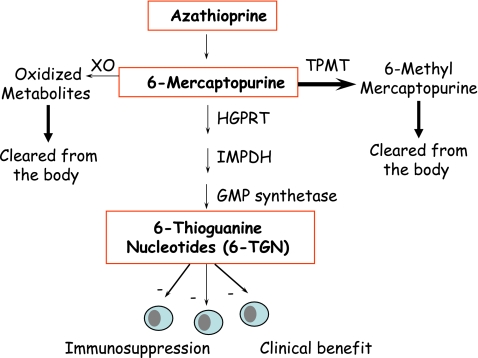
AZA is a prodrug, and is converted in the body by a series of steps to metabolites 6-mercaptopurine (6-MP) and 6-thioinosinic acid, and subsequently to the active metabolites 6-thioguanine (6-TGN; Figure 1). The 6-TGN formed in vivo from AZA inhibit purine synthesis by directly competing with the purine nucleotide guanosine. Purines are essential for the proliferation of cells, especially leukocytes and lymphocytes that are relatively deficient in purine synthesis and thus more sensitive than other cell types to the DNA synthesis inhibitory capacity of AZA. Another reason that these cells are more sensitive to AZA is the low expression levels of xanthine oxidase, which in other cell types converts some of the AZA to nontoxic metabolites. AZA is, thus, an effective immunosuppressive drug when used alone or in polytherapy. However, the use of thiopurine drugs is limited by toxicity including hematological adverse events, hepatotoxicity, and pancreatitis. The most severe side effect is bone marrow suppression, and extra caution including regular monitoring of the patients blood cell counts is recommended through the drug package insert, national formularies, and professional society guidelines.4
Figure 1.
A scheme showing the metabolic pathways for azathioprine. HGPRT, hypoxanthine guanine phosphoribosyltransferase; IMPDH, inosine monophosphate dehydrogenase; XO, xanthine oxidase. Adapted from Wang and Weinshilboum7 by Adva Levy-Barda.
In a series of patients treated over a 27-year period (1964–1991) with 2 mg/kg per day of AZA, there was drug-attributable myelosuppression in 5%, including severe leukopenia in 1.2% of patients.8, 9 Of these, 1.2% of leukopenic patients (n=9), three had pancytopenia and two died. Thus, although rare, severe hematological toxicity can occur and can be fatal in about 0.3% (2 of 739) patients.
In addition, hepatotoxicity is common among AZA-treated patients. A recent Spanish study detected abnormal liver enzyme levels in 15% and hepatotoxicity in 5% among 786 adult IBD patients prescribed with AZA.10 The data were corroborated by a recent US study in which hepatotoxicity was observed in 4.6% of 173 AZA-treated adult IBD patients.11 However, unlike myelosuppression, hepatotoxicity in AZA-prescribed patients was not correlated with thiopurine S-methyltransferase (TPMT) deficiency.12
Thiopurine S-methyltransferase
Thiopurine S-methyltransferase is a biotransformation phase II enzyme, responsible for the metabolic conversion of thiopurine drugs to inactive metabolites. Thus, heritable deficiency of TPMT activity places an individual at increased risk for myelosuppression that may be fatal.9, 7, 13, 14, 15
TPMT enzyme activity in the population is highly variable. Current data suggest that about 90% of individuals have high/normal activity of more than 10 U/ml blood (reflecting two active alleles), about 10% have intermediate TPMT activity of between 5 and 10 U/ml blood (reflecting only one active allele), and ∼0.3% (about 1 person in 300) have low/absent activity due to two inactive alleles.7 The latter are defined as having less than 5 U/ml blood of TMPT activity and are commonly referred to as ‘poor TPMT metabolizers'. To date, at least 23 TPMT alleles have been classified, including TPMT*1 (wild-type allele), which encodes the enzyme with normal activity.7 Three variant alleles TPMT*2, TPMT*3A, and TPMT*3C account for most of intermediate or low activity cases with allele frequencies of approximately 0.5, 5, and 0.5%, respectively, in Caucasians.
Notable differences in the frequencies of ‘poor TPMT metabolizers' were found among different ethnic groups.16, 17, 18 Although TPMT*3A is the most common inactive allele among Caucasians, in Africans the most common inactive allele is TPMT*3C. A recent study has shown that TPMT*3C is also the most common defective TPMT allele among Asians; it was present in 4.7% of Chinese, 4.4% of Malays, and 2.7% of Indians.19
TPMT was featured in the 2006 European Commission Joint Research Centre (JRC) IPTS report on ‘Pharmacogenetics and pharmacogenomics: state-of-the-art and potential socio-economic impacts in the EU'.20 The case study compared the cost-effectiveness for TPMT testing (by either phenotyping or genotyping) in children with acute lymphoblastic leukemia (ALL) treated with 6-MP in the UK, Germany, Ireland and the Netherlands, and concluded that TPMT testing was cost-effective for this particular indication. The present paper describes a follow-up European Commission (EC) study undertaken to study the extent and clinical utility of TPMT testing in AZA-treated patients in the UK and Spain.
Methods
The present study was designed as a follow-up and expansion of the 2006 JRC-IPTS study (http://ipts.jrc.ec.europa.eu/publications/pub.cfm?id=1387). Here, the focus is TPMT testing for RA and IBD in adults receiving AZA, in contrast to ALL featured in the previous EC study, where most patients were young children. The study brought together a team of experts in pharmacogenetics, pharmacology, and health economics. The study aims to review the literature and also summarizes the current evidence to support TPMT testing and supplements the literature review with opinions from experts in the field of TPMT testing. Two of these experts from the UK and Spain (WN and JPG, respectively) also contributed new data from patient cohorts in their own respective countries. The patient cohorts consisted 22 098 individuals undergoing clinical TPMT testing before thiopurine treatment in the UK, and 14 545 similar patients in Spain. An additional patient cohort in Spain included 7046 patients with IBD. The ultimate study aim was to ascertain data availability for performing cost-effectiveness studies in pharmacogenetics, through the collection of comparative data about the extent of AZA use and TPMT testing (either phenotypic or genotypic) in the UK and Spain as case studies.
Results
Use of azathioprine in the UK and Spain
The estimated number of AZA-treated patients in the UK each year is between 30 000 and 40 000 (WN, personal communication). Estimates were not available for Spain. However, information supplied by IMS (Spain) indicates that the number of AZA packs (50 tablets of 50 mg) sold during 2006 was 411 769 at a total cost of €2 977 865.
Assuming an average maintenance dosage of 150 mg (2 mg/kg) per day, as recommended by WHO (http://www.whocc.no/atcddd/), this sales volume may be translated to at least 18 800 patients (if taken over the entire year) and figures are likely to be much higher, as the initial dosage is typically 50 mg per day and presumably some patients did not take AZA for the entire year.
AZA adverse drug reactions and TPMT testing in the UK
Estimates in the published literature for the frequency of mild leukopenia among AZA-treated individuals vary in the range of 5–25% of patients receiving the drug.1, 2, 3, 4, 5, 6 However, severe leukopenia was reportedly less frequent in a study of 396 IBD patients who were receiving 6-MP, 2% of the cases developed clinical severe bone marrow suppression that required hospitalization.21
Examination of the online records for reports on adverse drug reactions (ADRs) in patients prescribed AZA on the UK Medicines Control Agency website (http://www.mhra.gov.uk) provides a detailed account of AZA-related ADRs during the reporting period of 1 July 1963 to 19 May 2006. During this period, the UK Medicines Control Agency received 1030 ADR reports on AZA, and 110 of them were fatal. It is not clear how many of these 110 fatal cases were the direct result of prescribing AZA. However, a close examination of this AZA ‘Drug Analysis Print' document identifies numerous ADRs and related fatalities that seem a typical side-effect profile of AZA (Table 1).
Table 1. Notable azathioprine-related fatalities and serious ADRs reported to the UK Medicines Control Agency from 1 July 1963 until 19 May 2006.
| Adverse event | Reported events | Reported fatalities |
|---|---|---|
| (A) All causes | 1030 | 110 |
| (B) Leading serious adverse events associated with fatalities | ||
| Pancytopenia | 55 | 3 |
| Thrombocytopenia | 32 | 2 |
| Leucopenia | 24 | 2 |
| Bone marrow depression | 24 | 2 |
| Agranulocytosis | 16 | 2 |
| Aplastic anemia | 14 | 5 |
| Sepsis | 11 | 4 |
| Granulocytopenia | 8 | 1 |
| Hepatic failure | 5 | 5 |
| Febrile neutropenia | 2 | 1 |
On the basis of the UK Medicines Control Agency AZA report, one may estimate that the rate of fatal ADRs among AZA users is in the range of 1 per 10 000 patients. This estimate is based on an assumption of about 1 million Britons who were prescribed AZA during the report period of 1963–2006. There are two main reservations to this estimate, first, the trend may be toward less AZA-related ADRs in more recent years, as it is likely that awareness of AZA-related ADRs has improved over time; moreover treatment of leukopenia and related sepsis has improved in more recent years so one may expect AZA-related fatalities to be reduced. However, an analysis performed by the UK Medicines Control Agency (September 2007) on our request has shown that this was not the case: for the period of 1 July 1963 to 31 December 1989, there have been 272 AZA-related ADR reports of which 30 were fatal, whereas for the period of 1 January 1990 to 31 August 2007 there have been 853 such reports of which 90 were fatal (detailed reports available on request). Second, underreporting of ADRs is known to be a global problem. A recent large meta-analysis of 37 studies, using a wide variety of surveillance methods, concluded that on average only about 5% of ADRs are being reported.22 Thus, true UK statistics of AZA-related ADRs may be up to 20-fold higher than those reported to the UK Medicines Control Agency.
In the UK, TPMT genotyping is unavailable as part of routine clinical practice. TPMT phenotyping, on the other hand, is available routinely to clinicians through two laboratories; since 1990 from the Purine Research Laboratory, Guy's Hospital, London and more recently, since 2002, from the Clinical Biochemistry, City Hospital, Birmingham. The Guy's laboratory currently uses a tandem mass spectrometry method of TPMT phenotyping, whereas the Birmingham laboratory employs whole-blood HPLC technique.
In 2006, the Guy's laboratory performed 10 931 TPMT assays (Dr A Marinaki, personal communication) whereas the Birmingham laboratory performed 11 167 tests in the calendar year from April 2006 to April 2007.23 Therefore, these two laboratories may be providing pretreatment TPMT testing in about 50% of individuals prescribed with AZA in the UK. Over these reporting periods Guy's laboratory identified 40 deficient patients (1/275 cases) whereas the Birmingham laboratory identified 70 deficient patients (1/169 cases) and, in addition, 1493 (2/15) individuals with intermediate activity. Costs for TPMT testing and calculated average costs for identifying one TPMT-deficient individual are presented in Table 2.
Table 2. Comparing frequencies for TPMT deficiency and costs per identified TPMT-deficient individual in the UK and Spain.
| Country/Site | Method | Period | No. of tests | TPMT deficiency rate | Cost per sample | Average cost per identified TPMT-deficient individuala |
|---|---|---|---|---|---|---|
| UK | ||||||
| Guy's Hospital, London | Phenotyping/MS | January 2006–December 2006 | 10 931 | 1:275 | £30 | £8250 |
| City Hospital, Birmingham | Phenotyping/HPLC | April 2006–April 2007 | 11 167 | 1:169 | £28 | £4732 |
| UK combined (both locations) | 22 098 | 1:201 | £29 | £5829 | ||
| Spain | ||||||
| UCB Pharma | Phenotyping/HPLC | January 2006–December 2006 | 5372 | 1:200b | €40 | €8000 |
Abbreviations: HPLC, high-performance liquid chromatography; MS, tandem mass spectrometry, TPMT, thiopurine S-methyltransferase.
Figures are shown and calculated only for TPMT-deficient individuals and do not include those with intermediate TPMT values (apparently those being heterozygous for TPMT) who may also be at higher risk for AZA-related adverse events.
Figures on TPMT deficiency in Spain are estimated from Gisbert et al.24
Genotyping was performed in both the Guy's and the Birmingham laboratories. In Guy's laboratory this was undertaken on a research basis by the RFLP technique for TPMT*3C, TPMT*3A, and TPMT*2 alleles; whereas 902 genetic tests were performed in the Birmingham laboratory for various indications, including to confirm undetectable TPMT levels and in patients who have undergone a blood transfusion within the previous 3 months. TPMT phenotyping following blood transfusion may lead to ‘false negative' in a poor TPMT metabolizer due to the TPMT activity of the donor's erythrocytes, whereas genotyping (based on DNA in the patient's peripheral blood lymphocytes) is not affected by blood transfusions. Genotyping is also undertaken on samples from patients who have experienced an ADR due to AZA, where there is uncertainty about the phenotype.
Genetic testing for the three common TPMT variant genotypes (TPMT*2, TPMT*3A, and TPMT*3C) was offered in the UK by the National Genetics Reference Laboratory (also CPA approved) at Manchester, as part of a Department of Health funded prospective randomized controlled trial (RCT) – TPMT: azathioprine response to genotyping and enzyme testing (TARGET) from 2005 to 2008 (http://www.nowgen.org.uk/stories/199-target). The estimated charge for genotyping using either TaqMan or pyrosequencing technologies is £30 per sample but, one may expect that genotyping costs will be sharply reduced in future.
AZA adverse drug reactions and TPMT testing in Spain
AZA is also widely prescribed in Spain for the same range of indications as in the UK. A recent Spanish study25 prospectively evaluated whether the choice of AZA or 6-MP dose in 131 IBD patients, based on TPMT activity, prevented myelotoxicity. Among the four patients (3%) having myelotoxicity, one had intermediate basal TPMT levels and three had high TPMT levels, but no patient had low TPMT levels. Therefore, this relatively small prospective study could not confirm that the choice of AZA or 6-MP, based on TPMT activity, prevents myelotoxicity in patients with IBD. Nevertheless, as no patient with low TPMT activity (<5 U/ml) was treated with AZA based on these studies, it is not possible to conclude if TPMT deficiency can predict AZA-related myelotoxicity.
Comparable to the UK, in Spain TPMT testing is rarely performed by genotyping but frequently performed by the HPLC phenotypic method.26 The cost for determining TPMT by this method is €40 per determination. Assuming a rate of 1:200 for TMPT deficiency24 one arrives at a high-end average cost of €8000 per identified TPMT-deficient individual for Spain (Table 2). Wider application of TPMT testing in Spain would likely lead to substantially reduced costs. Indeed, UCB Pharma, the laboratory responsible for the TPMT testing in Spain, reduced its charges from €80 to €40 per determination in the summer of 2007. The number of TMPT tests performed in Spain during 2006 is stated to be 5372, which is roughly three times less frequently compared to the UK assuming closely similar percentage of AZA prescriptions in the population and considering the 1.5-fold larger population of the UK compared with Spain.
Discussion
TMPT testing has become increasingly used by UK clinicians. In Spain, TPMT testing is less common compared with the UK but its use is rapidly expanding, thanks to more recent coverage by the Social Security System. The dramatic increase of TPMT testing in the UK in recent years is remarkable. Despite the emergent literature throughout the 1980s and 1990s regarding TPMT activity and ADRs to thiopurines, testing was very limited in the UK until the turn of the century. In 1997, a survey of 370 UK dermatologists reported that none used TPMT enzyme testing before treatment with AZA.27 By 2002, the Purine Research Laboratory at Guy's Hospital, London reported that dermatologists had requested 1479 TPMT assays between 1990 and 2000.28 According to a UK survey, conducted in 2005, TPMT phenotypic testing was used by 68% of respondents, with more dermatologists (95%) using testing compared to gastroenterologists (60%) and rheumatologists (49%).29 The reasons for this lag in uptake were multifold and probably reflected a lack of knowledge about the utility of testing, a lack of evidence to support routine testing, a lack of clinical testing services, concerns about costs, concerns that TPMT activity did not predict all episodes of leukopenia,30 and the fact that TPMT testing would not obviate the need for routine post-treatment monitoring.
Genotypic TPMT testing is limited, at this stage, in both the UK and Spain for research only. TPMT genotyping is mostly used for confirming poor TMPT metabolizer phenotypic test results or for testing individuals who received a blood transfusion during the last 3 months and for whom a phenotypic test could yield a false-negative result. Currently, the main drawbacks of genotypic TPMT testing are lack of standardized testing and lack of knowledge about the phenotypic correlates of some of the rare TPMT mutations, in particular among non-Caucasians for whom less knowledge about the TPMT polymorphism is available.
Although TPMT enzyme testing is now part of established practice, some uncertainties remain in the evidence base supporting the routine use of TPMT testing. These uncertainties primarily reflect poor knowledge on true rates for fatal or life-threatening AZA-related ADRs and whether the introduction of TPMT testing has had an effect on these rates. TPMT testing, as sensitive and as cheap as it might become in future, will never be able to identify, in advance, all patients who might suffer severe AZA-related ADRs, including severe leukopenia. For these reasons, among others, TPMT testing alone cannot assure maximal safety for AZA- or 6-MP-prescribed patients, and regular monitoring of blood counts and liver functions must be continued, even in patients who are found to be ‘normal TPMT metabolizers'.
Notably, in some sub-Saharan regions the most common inactive allele is TPMT*8 (which is absent in Caucasians and Asians) and the overall frequency of poor TMPT metabolizers is 1% of the population – about threefold higher than values, reported to date, for Caucasian or Asian populations.31 A new study32 testing TPMT activities in 1000 individuals, attending an inner-city hospital phlebotomy service in Birmingham (UK), over half of them non-Caucasians, has detected 0.6% patients with deficient TPMT activity, about twice compared with previous studies. These recent studies highlight that understanding the ethnic diversity of TPMT will be crucial for implementing its use in the clinic with genotype-based testing.
It is important to bear in mind that AZA-related adverse events are not necessarily the result of TMPT deficiency and may reflect drug interactions or variants of other metabolic enzymes, implicated in the metabolism of thiopurines, such as GST-M1. Indeed, Stocco et al33 have recently reported that IBD patients with a wild-type GST-M1 genotype present increased probability of developing adverse effects and increased incidence of lymphopenia during AZA treatment. However, the above study was small (n=70) and awaits corroboration by more comprehensive studies. Another enzyme implicated in AZA metabolism is inosine triphosphate pyrophosphatase (ITPA). A recent prospective British study in IBD patients (n=207) found that similar to TPMT deficiency, ITPA deficiency (both determined by genotyping) was also a predictor of AZA adverse effects.34 Notably, this study also reported that carrying a heterozygous TPMT genotype strongly predicted adverse effects (79% heterozygous vs 35% wild-type TPMT, P<0.001). These observations suggest that taking into considerations the benefits for carriers of heterozygous TPMT alleles may substantially improve the overall cost-effectiveness of TPMT genotyping for patients prescribed AZA. Similar findings on the contribution of ITPA deficiency to AZA myelotoxicity were observed in a larger US cohort (n=490) of children with ALL.35 Moreover, there was a significantly higher probability of severe neutropenia in patients with a variant ITPA allele whose dose of 6-MP had been adjusted for TPMT genotype; however, for patients whose 6-MP dose was not adjusted for TPMT phenotype, the TPMT genotype had a greater effect than the ITPA genotype.35 Thus, in future it may be necessary to test not only TPMT, but also ITPA, either by genotypic or phenotypic assays, as part of the thiopurine treatment protocol. Polymorphic alleles of genes implicated in DNA methylation may also affect the safety and efficacy of thiopurine drugs.36
TPMT testing could serve as a useful guide for developing sufficient evidence to inform decision-makers on whether to introduce a pharmacogenetic test into practice. This study has shown that retrospective studies alone are not sufficient and further prospective evidence is required to understand the potential for a pharmacogenetic test, to predict accurately the risk of a side effect or chance of a good response. Therefore, timely, well-designed RCTs of pharmacogenetic tests are vital pieces of information to add to the evidence base for the introduction of future tests.37 The TARGET study is such an RCT that will allow an in-depth comparison of genotyping and phenotyping approach to the assessment of the patient TPMT status.38
Clearly, an RCT must be timely to provide clinicians and other decision-makers with the evidence when needed. When the TARGT study was started in 2005, the uptake of TPMT testing was less widespread. However, the fact that TPMT phenotyping is so well established in the UK makes it unlikely that a different strategy will be adopted, unless compelling evidence is forthcoming. Importantly, despite the exponential increase in the uptake of TPMT testing in the UK, the number of ADRs that may have been avoided, by this increased uptake in TPMT testing and its use in clinical practice in the UK, remains unknown as the outcome data are not routinely collected by clinical service laboratories.
This paper does not discuss the economic implications of TPMT testing, which are reported elsewhere39 as part of the overall above-mentioned JRC study. Current laboratory charges in the UK and Spain were used to provide estimates of the average costs per identified TPMT-deficient individual and serve as a broad comparison between the two countries (Table 2). The analysis indicated very similar costs to identify a TPMT-deficient individual in the UK and Spain. The charge paid by a health-care service does not generally represent the true cost of providing the service. A pharmacogenetic test must be funded from finite health-care budgets and it is necessary to consider the opportunity cost of diverting resources toward routine TPMT testing services. In addition to the cost of the test itself, the impact on other health-care resources, such as managing ADRs and frequency of blood monitoring, needs to be quantified. Currently, the costs of AZA-related ADRs and their management are not available. This pertains not only to the UK and Spain; costs for AZA-related ADRs have never been reported in the medical literature. To perform a robust economic evaluation, it is necessary to identify and quantify the costs and benefits for alternative management strategies. The types of costs to consider, in such an analysis, are driven by the perspective of the decision-maker who is responsible for how to spend a given budget. Outcome data should measure the impact on the patient. There are no data available that describe the impact of TPMT testing on key patient outcomes, such as mortality and health-related quality of life. A summary of limitations in the TPMT cost-effectiveness studies to date is presented in Box 1. These missing data are necessary for improved cost-effectiveness analyses, which will provide valuable information for policy-makers about the impact of TPMT testing on health-care costs and patient outcomes and whether the routine introduction of TPMT testing has added value, compared with current practice.
Box 1. A summary of limitations in the TPMT cost-effectiveness studies to date.
| • A lack of high-quality data on which to base input parameters |
| • Differences in assumptions about the dosing regimen for AZA, the alternatives screening strategies, and study population included in the analysis |
| • Uncertainty as to whether an incremental analysis of TPMT testing compared to phenotyping or usual care had been conducted |
| • Lack of a direct prospective comparison of the clinical utility and costs of providing genotype-based testing with phenotype testing |
| • Uncertainty as to whether the treatment pathways reflected the biological process of the disease in question and the true impact of the intervention under evaluation |
| • Lack of accurate modeling of current clinical practice when AZA is prescribed and monitored |
| • Lack of analysis of the impact of assumptions made about the model structures (structural uncertainty) or the impact of systematic differences between patient subgroups (heterogeneity) |
| • Lack of measures of the impact of pretreatment testing on patients' health-related quality of life |
Conclusions
In closing, it seems appropriate to cite from the April 2007 report by the US HTE Policy Roundtable Panel:
The field of genetics shows great promise relative to understanding and using data on Heterogeneity of Treatment Effects (HTE), and genetic correlates of responsiveness and vulnerability should be investigated. This is especially important in terms of adverse drug reactions. Yet the issue of cost-effectiveness cannot be overlooked. Even if reliable genetic tests are developed that can accurately predict which patients will or will not experience adverse effects, it may be too costly to use such tests. Further research, particularly to identify genetic polymorphisms associated with patient response and vulnerability to side effects, is needed to evaluate variations in heterogeneous populations.40
Similarly, a more recent report (May 2008) by the US Department of Health and Human Services (HHS) Secretary's Advisory Committee on Genetics, Health, and Society41 states that ‘Successful translation of PGx tests and therapies into clinical practice and public health and policy likely will depend on a demonstration of their cost-effectiveness relative to standards of care.' The latter report includes in its specific recommendations that ‘HHS should identify and address evidence gaps in the analytical and clinical validity, clinical utility, cost-effectiveness, and value of PGx technologies'.
We firmly agree with these timely recommendations. This study has found that TMPT enzyme (ie, phenotypic) testing has become increasingly used by UK and Spanish clinicians but TPMT genotyping is not yet part of routine practice. Retrospective studies have shown an association between TPMT status and the risk of myelosuppression among AZA-treated patients. However, further evidence is necessary to provide a complete picture of the impact of introducing routine TPMT testing for these patients and its cost-effectiveness. Key data include evidence in favor of TMPT testing in addition to monitoring of AZA-prescribed patients, as a tool for reducing the number of episodes of myelosuppression, averting deaths from neutropenic sepsis, and improving health-related quality of life.
Acknowledgments
We dedicate the article to the memory of Emma Gutierrez de Mesa. The paper is based on the 2007 European Commission's JRC study ‘Improving pharmacovigilance in Europe: cost-effectiveness analysis of CYP2D6 and TPMT genotyping in general clinical practice'. We thank Kathryn Phillips (University of California, USA) for valuable discussions, Adva Levy-Barda (Faculty of Medicine, Tel-Aviv University, Israel) for the help provided in adapting figures and Ms Jenny Wong from the Vigilance and Risk Management of Medicines unit at the UK Medicines and Healthcare Products Regulatory Agency for carrying out time-based analysis for Azathioprine ADRs and fatality reports on our request. Thanks to Dr Tony Marinaki, Purine Research Laboratory, London for data on TPMT testing services. The views expressed here are those of the authors and do not necessarily reflect the views of the European Commission.
Footnotes
Web links
101. Medline Plus advice on Imuran (a service of the National Institutes of Health)
http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682167.html
102. UK Medicines Control Agency
References
- Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46:187–208. doi: 10.2165/00003088-200746030-00001. [DOI] [PubMed] [Google Scholar]
- Coulthard S, Hogarth L. The thiopurines: an update. Invest New Drugs. 2005;23:523–532. doi: 10.1007/s10637-005-4020-8. [DOI] [PubMed] [Google Scholar]
- Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731–743. doi: 10.1016/s1542-3565(04)00344-1. [DOI] [PubMed] [Google Scholar]
- Timmer A, McDonald JW, Macdonald JK.Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis Cochrane Database Syst Rev 2007. CD000478. [DOI] [PubMed]
- Massacesi L, Parigi A, Barilaro A, et al. Efficacy of azathioprine on multiple sclerosis new brain lesions evaluated using magnetic resonance imaging. Arch Neurol. 2005;62:1843–1847. doi: 10.1001/archneur.62.12.1843. [DOI] [PubMed] [Google Scholar]
- Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006;55:369–389. doi: 10.1016/j.jaad.2005.07.059. [DOI] [PubMed] [Google Scholar]
- Wang L, Weinshilboum R. Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene. 2006;25:1629–1638. doi: 10.1038/sj.onc.1209372. [DOI] [PubMed] [Google Scholar]
- Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–1085. doi: 10.1136/gut.34.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clunie GP, Lennard L. Relevance of thiopurine methyltransferase status in rheumatology patients receiving azathioprine. Rheumatology (Oxford) 2004;43:13–18. doi: 10.1093/rheumatology/keg442. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Luna M, Gonzalez-Lama Y, et al. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106–1114. doi: 10.1002/ibd.20160. [DOI] [PubMed] [Google Scholar]
- Shaye OA, Yadegari M, Abreu MT, et al. Hepatotoxicity of 6-mercaptopurine (6-MP) and azathioprine (AZA) in adult IBD patients. Am J Gastroenterol. 2007;102:2488–2494. doi: 10.1111/j.1572-0241.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- Zelinkova Z, Derijks LJ, Stokkers PC, et al. Inosine triphosphate pyrophosphatase and thiopurine s-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin Gastroenterol Hepatol. 2006;4:44–49. doi: 10.1016/j.cgh.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Baker DE. Pharmacogenomics of azathioprine and 6-mercaptopurine in gastroenterologic therapy. Rev Gastroenterol Disord. 2003;3:150–157. [PubMed] [Google Scholar]
- Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149–1157. doi: 10.1111/j.1440-1746.2005.03832.x. [DOI] [PubMed] [Google Scholar]
- Boonsrirat U, Angsuthum S, Vannaprasaht S, et al. Azathioprine-induced fatal myelosuppression in systemic lupus erythematosus patient carrying TPMT*3C polymorphism. Lupus. 2008;17:132–134. doi: 10.1177/0961203307085255. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- Engen RM, Marsh S, Van Booven DJ, McLeod HL. Ethnic differences in pharmacogenetically relevant genes. Curr Drug Targets. 2006;7:1641–1648. doi: 10.2174/138945006779025446. [DOI] [PubMed] [Google Scholar]
- Jones TS, Yang W, Evans WE, Relling MV. Using HapMap tools in pharmacogenomic discovery: the thiopurine methyltransferase polymorphism. Clin Pharmacol Ther. 2007;81:729–734. doi: 10.1038/sj.clpt.6100135. [DOI] [PubMed] [Google Scholar]
- Kham SK, Soh CK, Liu TC, et al. Thiopurine S-methyltransferase activity in three major Asian populations: a population-based study in Singapore. Eur J Clin Pharmacol. 2008;64:373–379. doi: 10.1007/s00228-007-0426-x. [DOI] [PubMed] [Google Scholar]
- van den Akker-van Marle ME, Gurwitz D, Detmar SB, et al. Cost-effectiveness of pharmacogenomics in clinical practice: a case study of thiopurine methyltransferase genotyping in acute lymphoblastic leukemia in Europe. Pharmacogenomics. 2006;7:783–792. doi: 10.2217/14622416.7.5.783. [DOI] [PubMed] [Google Scholar]
- Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- Payne K. Towards an economic evidence base for pharmacogenetics: consideration of outcomes is key. Pharmacogenomics. 2008;9:1–4. doi: 10.2217/14622416.9.1.1. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Gomollon F, Cara C, et al. Thiopurine methyltransferase activity in Spain: a study of 14 545 patients. Dig Dis Sci. 2007;52:1262–1269. doi: 10.1007/s10620-006-9119-z. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Luna M, Mate J, Gonzalez-Guijarro L, Cara C, Pajares JM. Choice of azathioprine or 6-mercaptopurine dose based on thiopurine methyltransferase (TPMT) activity to avoid myelosuppression. A prospective study. Hepatogastroenterology. 2006;53:399–404. [PubMed] [Google Scholar]
- Kroplin T, Weyer N, Gutsche S, Iven H. Thiopurine S-methyltransferase activity in human erythrocytes: a new HPLC method using 6-thioguanine as substrate. Eur J Clin Pharmacol. 1998;54:265–271. doi: 10.1007/s002280050457. [DOI] [PubMed] [Google Scholar]
- Ford L, Prout C, Gaffney D, Berg J. Whose TPMT activity is it anyway. Ann Clin Biochem. 2004;41:498–500. doi: 10.1258/0004563042466866. [DOI] [PubMed] [Google Scholar]
- Holme SA, Duley JA, Sanderson J, et al. Erythrocyte thiopurine methyl transferase assessment prior to AZA use in the UK. QJM. 2002;95:439–444. doi: 10.1093/qjmed/95.7.439. [DOI] [PubMed] [Google Scholar]
- Fargher EA, Tricker K, Newman W, et al. Current use of pharmacogenetic testing: a national survey of thiopurine methyltransferase testing prior to azathioprine prescription. J Clin Pharm Ther. 2007;32:187–195. doi: 10.1111/j.1365-2710.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Ansari A, Hassan C, Duley J, et al. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1743–1750. doi: 10.1046/j.1365-2036.2002.01353.x. [DOI] [PubMed] [Google Scholar]
- Oliveira E, Quental S, Alves S, Amorim A, Prata MJ. Do the distribution patterns of polymorphisms at the thiopurine S-methyltransferase locus in sub-Saharan populations need revision? Hints from Cabinda and Mozambique. Eur J Clin Pharmacol. 2007;63:703–706. doi: 10.1007/s00228-007-0310-8. [DOI] [PubMed] [Google Scholar]
- Cooper SC, Ford LT, Berg JD, Lewis MJ. Ethnic variation of thiopurine S-methyltransferase activity: a large, prospective population study. Pharmacogenomics. 2008;9:303–309. doi: 10.2217/14622416.9.3.303. [DOI] [PubMed] [Google Scholar]
- Stocco G, Martelossi S, Barabino A, et al. Glutathione-S-transferase genotypes and the adverse effects of azathioprine in young patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:57–64. doi: 10.1002/ibd.20004. [DOI] [PubMed] [Google Scholar]
- Ansari A, Arenas M, Greenfield S, et al. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:973–983. doi: 10.1111/j.1365-2036.2008.03788.x. [DOI] [PubMed] [Google Scholar]
- Stocco G, Cheok M, Crews K, et al. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009;85:164–172. doi: 10.1038/clpt.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth LA, Redfern CP, Teodoridis JM, et al. The effect of thiopurine drugs on DNA methylation in relation to TPMT expression. Biochem Pharmacol. 2008;76:1024–1035. doi: 10.1016/j.bcp.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Payne K, Newman W, Fargher E, et al. TPMT testing in rheumatology: any better than routine monitoring. Rheumatology (Oxford) 2007;46:727–729. doi: 10.1093/rheumatology/kel427. [DOI] [PubMed] [Google Scholar]
- Wadelius M, Pirmohamed M. Pharmacogenetics of warfarin: current status and future challenges. Pharmacogenomics J. 2007;7:99–111. doi: 10.1038/sj.tpj.6500417. [DOI] [PubMed] [Google Scholar]
- Payne K, Newman WG, Gurwitz D, Ibarreta D, Phillips KA. TPMT testing in azathioprine: a ‘cost-effective use of healthcare resources'. Personalized Med. 2009;6:103–113. doi: 10.2217/17410541.6.1.103. [DOI] [PubMed] [Google Scholar]
- McLaughlin MJ. HTE Policy Roundtable Panel. Healthcare policy implications of heterogeneity of treatment effects. Am J Med. 2007;120 4 Suppl 1:S32–S35. doi: 10.1016/j.amjmed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human (HHS) Services Secretary's Advisory Committee on Genetics, Health, and Society (SACGHS) Report: realizing the potential of pharmacogenomics: opportunities and challenges(May 2008). http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_PGx_report.pdf .