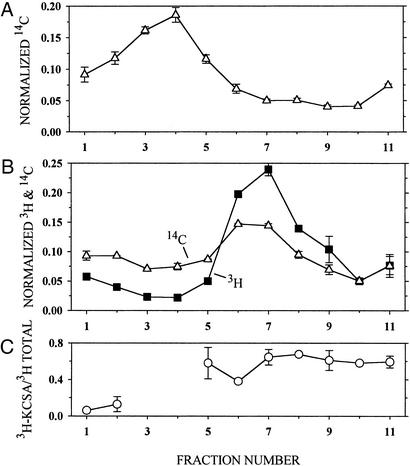
Figure 2.
Association of KcsA and phospholipid in reconstituted liposomes. Liposomes consisting of asolectin and 14C-phospholipid, suspended in KCa buffer, were added to DM/KCa buffer (A) or to 3H-labeled KcsA in DM/KCa buffer (B) and reconstituted by dilution. The concentrations of asolectin, protein, and DM and the reconstitution conditions were the same as those given in Materials and Methods. The mixture of asolectin and 14C-phospholipid was made by mixing 150 μl of 20 mg of asolectin per ml of CHCl3 and 300 nCi (1 Ci = 37 GBq) of 14C-dipalmitoyl phosphatidylcholine (25 nCi/μl of toluene/ethanol, 1:1). 3H-labeled KcsA was made by reacting E. coli inner membrane containing 25 μg of KcsA mutant K14C and 162 μg of total protein with 10 mM DTT (pH 8.0) for 60 min. This suspension was sedimented, and the pellet was suspended in 20 mM Hepes (pH 7.0)/1 mM EDTA, sedimented, and suspended again to lower the DTT concentration. The reduced protein was reacted with 4.4 μM 3H-NEM/90 μM NEM for 30 min and sedimented and suspended three times to lower the free 3H-NEM concentration. In duplicate, 200 μl of reconstituted liposomes containing 14C-phospholipid (A) and 200 μl of reconstituted liposomes containing 3H-KcsA and 14C-phospholipid (B) were layered over 4 ml of a linear gradient from 5–31% (wt/wt) sucrose in 10 mM Hepes (pH 7.0), and sedimented in a Beckman SW60 rotor at 60,000 rpm for 13 h at 4°C. The sucrose concentration and linearity of the gradient were determined in parallel tubes with an Abbe refractometer (Zeiss). Ten 400-μl fractions were taken from the top (fraction 1 is topmost), and the volume of the remaining 11th fraction, which was <400 μl, was determined. Forty-microliter aliquots of each fraction were mixed with 5 ml of scintillation fluid and counted with a double-label protocol. The average radioactivity (and average error), normalized by the total recovered radioactivity, is plotted versus the fraction number. (C) The part of the total 3H radioactivity that was associated with KcsA in each fraction in B was determined by diluting the remaining 360 μl of each fraction (less in fraction 11) with 0.5 ml of 1% DM/100 mM NaCl/50 mM NaPi (pH 8.0), stirring with 50 μl of NiNTA-agarose (1:1) for 60 min, washing the gel, and eluting the bound KcsA in two 50-μl aliquots of 5 mM EDTA/LSB (conditions as in Materials and Methods). The combined eluted 3H radioactivity was determined and is plotted versus the sucrose-density-gradient B fraction number normalized by the input to the NiNTA-agarose. There was too little 3H-labeled protein in sucrose-density-gradient fractions 3 and 4 to determine its binding to NiNTA-agarose.