Abstract
Menkes disease (MD) is a lethal multisystemic disorder of copper metabolism. Progressive neurodegeneration and connective tissue disturbances, together with the peculiar ‘kinky' hair are the main manifestations. MD is inherited as an X-linked recessive trait, and as expected the vast majority of patients are males. MD occurs due to mutations in the ATP7A gene and the vast majority of ATP7A mutations are intragenic mutations or partial gene deletions. ATP7A is an energy dependent transmembrane protein, which is involved in the delivery of copper to the secreted copper enzymes and in the export of surplus copper from cells. Severely affected MD patients die usually before the third year of life. A cure for the disease does not exist, but very early copper-histidine treatment may correct some of the neurological symptoms.
Keywords: Menkes disease, ATP7A, copper
In brief
Menkes disease (MD) is a lethal multisystemic disorder of copper metabolism.
Progressive neurodegeneration and connective tissue disturbances, together with the peculiar ‘kinky' hair, are the main manifestations.
MD is inherited as an X-linked recessive trait, and as expected the vast majority of patients are males.
MD occurs due to mutations in the ATP7A gene.
The vast majority of ATP7A mutations are intragenic mutations or partial gene deletions.
ATP7A is an energy-dependent, transmembrane protein, which is involved in the delivery of copper to the secreted copper enzymes and in the export of surplus copper from cells.
Severely affected MD patients die usually before the third year of life. A cure for the disease does not exist, but very early copper–histidine treatment may correct some of the neurological symptoms.
Introduction
Menkes disease (MD) is an X-linked multisystemic lethal disorder of copper metabolism. Patients usually exhibit a severe clinical course, with death in early childhood, but variable forms exist and occipital horn syndrome (OHS) is the mildest form. The defective gene in MD (ATP7A) is predicted to encode ATP7A, which is involved in the delivery of copper to the secreted copper enzymes and in the export of surplus copper from cells.
Normal and abnormal copper metabolism in human and other organisms has been the focus of extensive research, and tremendous knowledge has been accumulated on this subject. In this paper we will give a general view of MD and copper metabolism. Due to space restriction not all the original publications have been cited in this review, and relevant references can be found in two book chapters.1, 2
Clinical synopsis
MD shows considerable variability in its severity. Classical MD is the most severe form, while OHS is the mildest recognized form (reviewed by Tümer and Horn 2004, 20021, 2).
Classical MD
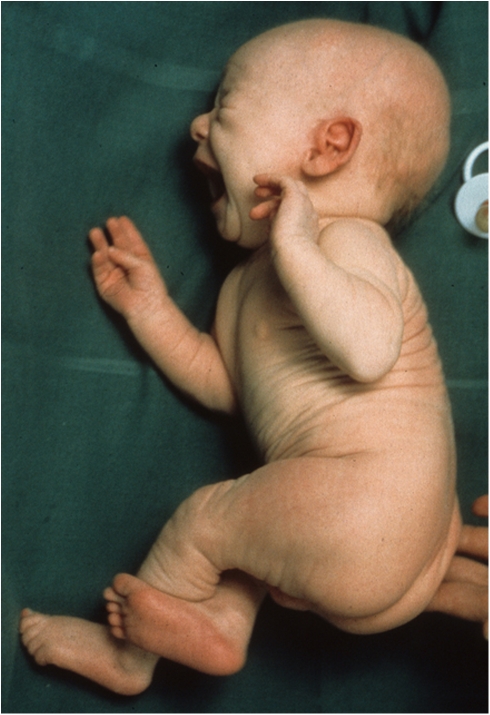
Progressive neurodegeneration and marked connective tissue dysfunction characterize the clinical picture of the most common severe form of MD, and death typically occurs before the third year of life (Figure 1).
Figure 1.
Clinical appearance at age 3 weeks of a patient with classical MD. Note the lax skin.
Pregnancy is usually uncomplicated. There may be premature labor and delivery, but most male patients are born at term with appropriate birth measurements. Cephalohematomas and spontaneous fractures are occasionally observed at birth. In the early neonatal period, patients may present with prolonged jaundice, hypothermia, hypoglycemia and feeding difficulties. Pectus excavatum and umbilical and inguinal hernias have also been reported. The first sign of MD may be unusual sparse and lusterless scalp hair that becomes tangled on the top of the head at the age of 1–2 months (Figure 2). At this time the appearance may be described as being odd, with pale skin, frontal or occipital bossing, micrognatia, pudgy cheeks, and a rather expressionless appearance. However, these changes are often too subtle to attract attention. Initial psychomotor development is usually unremarkable, with normal babbling and smiling up to about 2–4 months of age. The baby then ceases to develop further and gradually loses some of the previously developed skills. The developmental regression becomes obvious around 5–6 months of age. Most patients develop therapy-resistant seizures from about 2 to 3 months of age. Additional symptoms are failure to thrive, poor eating, vomiting, and diarrhea. Muscular tone is often decreased in early life, but is later replaced by spasticity and weakness of the extremities. As the motor dysfunction progresses, spontaneous movements become limited and drowsiness and lethargy emerge. The patients are typically diagnosed at 3–6 months of age, often due to the abnormal hair that is a striking feature of the disease. The hypopigmented or depigmented hair resembles and feels like steel wool; it is lusterless and friable, especially in the areas of the scalp subjected to friction.
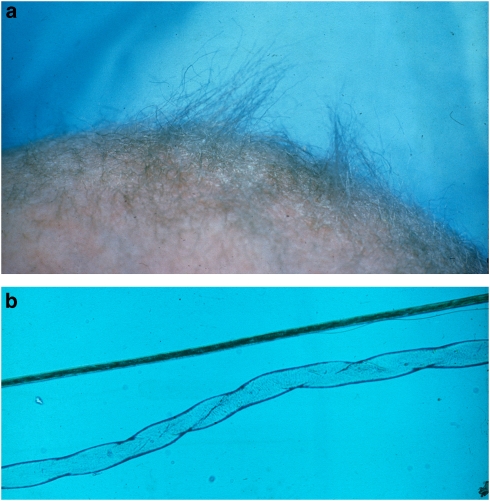
Figure 2.
Abnormal hair in a patient with classical MD. (a) Stubby appearance of depigmented scalp hair. (b) Hair microscopy ( × 100) of twisted hair shaft (pili torti) (below) and a normal hair strand (above) (courtesy of E Reske-Nielsen, Glostrup University Hospital, Denmark).
Vascular, urogenital, and skeletal abnormalities are numerous. Patients have skeletal changes, including pectus excavatum or pectus carrinatum, and spontaneous fractures due to generalized osteoporosis. The joints are hyperextensive, and loose and dry skin may be observed very early. Thick, scaly seborrheic dermatitis is also a frequent feature. Routine ophthalmoscopy is usually normal, but in later stages patients frequently fail to follow visual stimulus.
Late manifestations of the disease are blindness, subdural hematoma, and respiratory failure. Most patients die within the third year of life due to infection, vascular complications (such as sudden and massive cerebral hemorrhage due to vascular rupture), or from the neurological degeneration itself.
The OHS
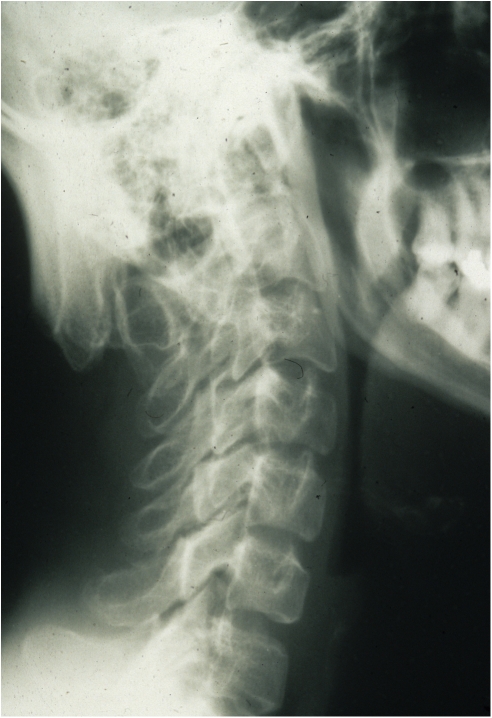
OHS is the mildest recognized form of MD, and its principal clinical features are related to connective tissue. The main distinction between OHS and the other forms of MD is the radiographic observation of characteristic occipital horns (Figure 3). These are symmetric exostoses protruding from the occipital bone and pointing down.
Figure 3.
Lateral skull radiograph of a 23-year-old OHS patient. The occipital exostoses (arrow) are not present at birth and become prominent by age (courtesy of I Kaitila, Helsinki University Hospital, Finland).
Pregnancy is usually normal. The skin may appear wrinkled and loose at birth, and umbilical or inguinal hernias may be present. Within days, hypothermia, jaundice, hypotonia, and feeding problems may develop. Clinical problems become gradually obvious, and the first signs that bring the child to medical attention may be intractable diarrhea or recurrent urinary tract infections. In spite of these problems, diagnosis of OHS is usually made only around 5–10 years of age.
Motor development is delayed due to muscular hypotonia and is associated with unusual clumsiness. Height is usually normal, while mild disproportion with long trunk, narrow chest and shoulders, thoracolumbar kyphosis or scoliosis, and pectus deformity are common. The joints are hypermobile. Elbow mobility is restricted and there is a tendency toward dislocation of the elbows. Facial appearance gradually becomes distinctive. Unusual features include long, thin face, often with a high forehead, down-slanting eyes, hooked or prominent nose, long philtrum, high arched palate, and prominent large ears. The extent of skin laxity is variable and may increase with age, resulting in droopy wrinkles around the trunk. Hair is usually not conspicuously abnormal, although some patients may have lusterless and unusually coarse hair. Recurrence of the inguinal hernia is common. Vascular anomalies, such as varicose veins, are common, and arterial aneurysms have also been described. A particular problem is orthostatic hypotension. The intellectual capacity is described as low to borderline normal. Pubertal development is normal.
The clinical course is characterized by chronic diarrhea, bladder diverticulae with recurrent urinary tract infections and occasional spontaneous bladder ruptures, orthostatic syncope, and joint instability in the inferior extremities and limitations at the elbows. Some patients require surgery for severe progressive thoracolumbar kyphosis, spontaneous retinal ablation, or mitral valve insufficiency. Life expectancy in OHS is variable, although substantially longer than that in MD. There are adult patients up to 50 years who have maternal male relatives dying in early childhood (I Kaitila, personal communication).
Intermediate phenotypes
A number of MD patients with milder symptoms and later onset have been described. However these intermediate forms are not well categorized and different descriptions such as mild, moderate, or long surviving MD have been used.
Normal and abnormal copper metabolism
Copper is the third most abundant trace element in the body, after iron and zinc, and is required for normal function of several copper enzymes participating in important metabolic processes (Table 1). Copper is involved in cellular respiration (cytochrome-c oxidase (COX)); neurotransmitter biosynthesis (dopamine β-hydroxylase); maturation of peptide hormones (peptidyl α-amidating enzyme); free-radical scavenging (superoxide dismutase); cross-linking of elastin, collagen (lysyl oxidase) and keratin (sulfhydryl oxidase); melanin production (tyrosinase); and iron homeostasis (ceruloplasmin and hephaestin). Copper has further been implicated in myelination in regulation of the circadian rhythm, and may also be necessary for coagulation and angiogenesis (reviewed by Tümer and Horn 2004, 20021, 2).
Table 1. Mammalian copper enzymes and their suggestive relationship between MD symptoms.
| Enzyme | Biological activity | Symptom |
|---|---|---|
| Cytochrome c oxidase | Cellular respiration | CNS degeneration Ataxia Muscle weakness Respiratory failure |
| Superoxide dismutase | Free radical scavenging | CNS degeneration |
| Ceruloplasmin | Iron and copper transport | Anemia |
| Hephaestin | Iron transport | Anemia |
| Tyrosinase | Pigment formation | Hypopigmentation |
| Dopamine β-hydroxylase | Catecholamine production | Ataxia Hypothermia Hypotension Diarrhea |
| Peptidyl α-amidating enzyme | Activation of peptide hormones | Wide spread effects |
| Lysyl oxidase | Collagen an elastin cross-linking | Premature rapture of fetal membranes Cephalohematoma Abnormal facies High-arched palate Emphysema Hernias Bladder diverticula Arterial aneurysms Loose skin and joints Osteoporosis Petechial hemorrhage Poor wound healing CNS degeneration |
| Sulfhydryl oxidase | Cross-linking of keratin | Abnormal hair Dry skin |
Abbreviations: CNS, central nervous system; MD, Menkes' disease.
Adapted from Horn and Tümer (2002).2
Although essential, owing to its chemical properties, the same metal may be highly toxic. Copper can exist in two oxidation states, Cu(I) and Cu(II), and reversible interchange between these two states is the basis of the enzymatic reactions. The same property, however, can result in the production of free radicals, which have detrimental effects on cellular components. Fine regulation of copper homeostasis is, therefore, vitally important for all living organisms.
Cellular copper metabolism
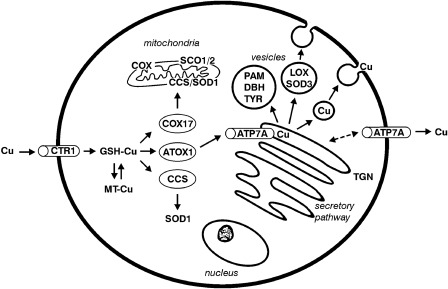
Copper uptake across the plasma membrane is likely to use an energy-independent membrane transporter (CTR1) (Figure 4).3 In the cytoplasma copper is bound to small proteins such as metallothionein4 and glutathione,5 or copper-specific chaperones, and thereby the cell is protected from the toxic effects of the free ion. The three known copper-chaperons, CCS, ATOX1, and COX17, bind the copper ion and guide it to different cellular locations, securing efficient delivery of the metal to the enzymes. CCS targets copper to superoxide dismutase, which resides in the cytosol or in mitochondria.6 COX17 guides copper to mitochondria to be incorporated into COX, where other proteins involved in COX copper metallation (such as COX11, SCO1 and SCO2) also reside (reviewed by Turski and Thiele7). ATOX1 guides copper to trans-Golgi network (TGN), where it is incorporated into copper-requiring enzymes synthesized in the secretory pathway.8
Figure 4.
Schematic illustration of cellular copper transport. Copper is taken up across the plasma membrane by the copper uptake transporter (CTR1) as cuprous ions (CuI). Within the cytoplasma, the copper is found attached to glutathione (GSH), metallothionein (MT), or copper chaperons, which deliver copper to enzymes and compartments. COX17 is the copper chaperone for COX, CCS is the copper chaperone for the cytoplasmic superoxide dismutase (SOD1), and HAH1 is the copper chaperone for ATP7A, which delivers copper to peptidyl-α-amidating enzyme (PAM), dopamine β-hydroxylase (DBH), tyrosinase (TYR), lysyl oxidase (LOX), and extracellular SOD3. ATP7A is also responsible for copper export from cells. At low copper concentrations, the localization of the protein is at the TGN, but at high copper concentrations it will be relocated to the plasma membrane. In the liver the role of ATP7A is performed by ATP7B.
In the TGN two homologous membrane-bound, copper-specific ATPases, ATP7A and ATP7B (defective in MD and Wilson disease, respectively), transfer copper across the membrane into the lumen of the TGN, where it is delivered to secreted enzymes. ATP7A is expressed in almost every organ except the liver where ATP7B is predominantly expressed. In concordance with this, copper is incorporated in ceruloplasmin by ATP7B in hepatocytes, while ATP7A is in charge in most other cell types in transporting copper to tissue-specific enzymes. This also reflects why MD is a systemic disorder, while in Wilson disease, mainly the liver is affected.
ATP7A (and ATP7B) has a dual role in the cell: apart from copper-loading of enzymes in the secretory pathway, it is responsible for ATP-driven efflux of copper from the cells.9 Under normal physiological copper concentrations ATP7A is localized to TGN, transporting copper into the lumen to the copper-dependent enzymes. Under increased copper concentrations ATP7A is translocated to the vesicles10 or to the plasma membrane.11
Whole-body copper metabolism
In mammals the essential source for copper is the diet and for humans the average intake is about 1 mg/day. The dietary copper is absorbed from the intestinal lumen across the mucosal barrier into the interstitial fluid, and to portal blood. The non-specific metal transporters, DMT1, ATP7A, and CTR1, are involved in this multistage process.12, 13
From the blood the copper is mainly transported to the liver and in lesser amounts to the kidney and other tissues including brain. In the liver, which is the central organ of copper storage and homeostasis, the copper is either secreted to the blood bound to ceruloplasmin or excreted to the bile. Both processes are controlled by ATP7B; defective ATP7B will, thus, lead to increased amounts of copper in the liver and other organs as observed in Wilson disease patients. The main excretory route of copper is bile and urinary loss is negligible.
Free copper ions are virtually non-existing in living organisms. The concentration of free copper ions has been estimated to be in the order of 10−13 in the human blood14 where the metal is mainly bound to ceruloplasmin, albumin, and histidine. Ceruloplasmin is the major copper-containing protein component in serum, but it is disputable whether it has a role in copper metabolism.15 Albumin-bound copper is in equilibrium with amino-acid-bound copper and these two forms probably constitute a buffer system that secures the availability of sufficient copper to tissues as well as protecting against copper toxicity.
The copper is transported to the brain across the blood–brain barrier at the cerebral endothelium and the blood–cerebrospinal fluid barrier at the choroid plexus. The details of copper transport to the brain are yet unknown. The copper transporters CTR1, ATP7A, and ATP7B are all expressed profoundly in brain barrier fractions, indicating a possible role of these transporters in brain copper uptake.16 In the brain ATP7A is involved in normal functioning of copper-dependent enzymes. Expression of ATP7A in the brain is developmentally regulated and it is high in the early postnatal period.17
Copper homeostasis in MD
Elimination of copper from cells is the basic disturbance in MD, and almost all the tissues except for liver and brain will accumulate copper to abnormal levels., Although high, the copper level does not reach a toxic state in MD. This is partly due to an already diminished intestinal copper absorption, because of defective copper export from the mucosal epithelium, and partly due to the scavenger role of metallothionein.
In the liver of MD patients, the low copper content is due to requirement of the metal in other tissues, rather than disturbed copper metabolism, as in the normal liver ATP7B, but not ATP7A, is the main copper transporter.
The reason for the low copper content in the brain of MD patients is however different. The mammalian brain is one of the richest copper-containing organs in the body. Regulation of brain copper level is not well understood, but ATP7A must participate in this process, since MD leads to low copper levels in the brain. In MD patients, copper is likely trapped in both the blood–brain barrier and the blood–cerebrospinal fluid barrier, while the neurons and glial cells are deprived of copper.18 This also supports the role of ATP7A in brain copper uptake. Neuronal demyelination is also observed in MD patients due to ATP7A inactivation.19 Mediation of ATP7A-related copper release through NMDA-receptor activation suggests a new role of this protein in brain dysfunction other than through deprivation of copper-dependent enzymes.20 It is, thus, likely that seizures and neuronal degeneration observed in MD patients may also be related to a disturbed neuronal transmission through impaired function of NMDA receptors.20
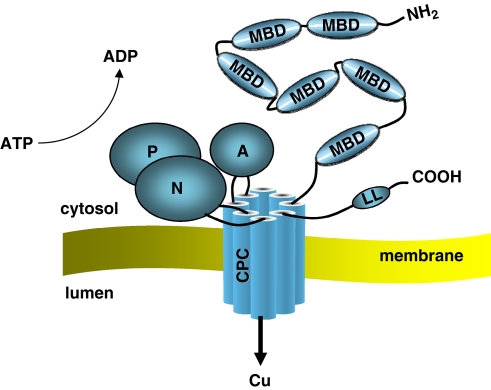
Structure of the ATP7A protein
ATP7A (and ATP7B) is a member of a large family of P-type ATPases that are energy-utilizing membrane proteins functioning as cation pumps (Figure 5) (reviewed by Lutsenko et al22). They are called ‘P-type' ATPases, as they form a phosphorylated intermediate during the transport of cations across a membrane. The super-family of P-type ATPases also includes the Na+/K+ and H+/K+ pumps, as well as plasma membrane and sarcoplasmatic reticulum Ca2+ pumps.21
Figure 5.
Schematic 3D protein structure of ATP7A with the functionally important domains. At the N-terminal ATP7A has six MBDs with the copper-binding motifs (CXXC). The protein is anchored to the membrane with the transmembrane domains (TMDs) and within TMD6 resides the CPC motif, which is assumed to play a direct role in copper translocation. The activation domain (A) with the invariant TGE residues, the nucleotide-binding domain (N), and the phosphorylation domain (P) with the invariant aspartate residue (D) are important domains for the catalytic activity of ATP7A.
ATP7A (and ATP7B) transports copper across a membrane using the energy released by hydrolysis of ATP. This process (catalytic activity) involves domains specific for binding and hydrolysis of ATP, and is similar in all P-type ATPases. The domains involved in the catalytic cycle of the protein are the nucleotide-binding domain (N-domain), phosphorylation domain (P-domain), and activation domain (A-domain). Transport and translocation of copper furthermore requires special motifs and structures for recognition, binding, and translocation of the metal across a membrane. These motifs contain cysteine (C) residues, which play an important role in copper binding.
At the N-terminus ATP7A has six metal-binding domains (MBD1–6) each with a consensus MTXCXXC motif. Copper binds to these domains in the reduced form, Cu(I). It is assumed that the two MBDs (MBD5 and MBD6) closest to the transmembrane domains are important for the functional activity of the protein, and at least one of these two sites is necessary for normal function of the protein. The first four metal-binding domains (MBD1–4) are thought to have a regulatory function. Interaction between ATP7A and the copper chaperone ATOX1 occurs through these domains.
ATP7A is anchored to a membrane through eight hydrophobic transmembrane domains, which form a channel for copper translocation through the membrane, and the CPC motif within TMD6 is assumed to play a direct role in the transfer of copper.
The catalytic activity of ATP7A is likely mediated through a coordinated action of the N-domain, the P-domain, and the A-domain. The N- and P-domains reside between TMD6 and TMD7. N-domain binds ATP and the γ-phosphate of ATP is then transferred to the invariant aspartate residue (D) in the DKTG motif, which resides in the P-domain. This results in the formation of a transient phosphorylated intermediate. Following translocation of the copper through the membrane, the P-domain is dephosphorylated. The A-domain is located between TMD4 and TMD5, and includes the invariant TGE motif, where the glutamate residue (E) plays a key role in dephosphorylation of the phosphorylated intermediate.
At the C-terminal ATP7A contains conserved di-leucine residues (LL), which is necessary for retrieval of the protein from the membranes (plasma membrane or vesicles).
Genetic basis of MD
The incidence of MD is calculated as 1 in 300 000 based on observations from a large population in five European countries,23, 24 and 1 in 360 000 in Japan.25 In Australia the incidence is reported to be much higher (1 in 50 000–100 000),26 but this might be due to a founder effect.
The 8.5-kb transcribed sequence of ATP7A is organized in 23 exons, which span a 140-kb genomic region27 (UCSC Genome Browser; http://genome.cse.ucsc.edu/). To date about 170 different mutations (from single-amino-acid substitutions to large deletions and chromosome aberrations) affecting ATP7A have been reported28, 29, 30, 31, 32 (Human Gene Mutation Database (HGMD); www.hgmd.org).
As expected the vast majority of MD patients are males, although a few female patients have also been reported. Most of the female patients have an X;autosome translocation, where the normal X-chromosome is preferentially inactivated.29 However, female patients with a point mutation and skewed inactivation of the normal X-chromosome have also been observed (unpublished data).
Chromosome abnormalities affecting ATP7A were detected in eight patients, one male33 and seven female patients. One of the female patients was mosaic for the Turner karyotype and the rest had X;autosome translocations (reviewed by Sirleto et al34). Approximately 25% of the ATP7A mutations (n=50) are gross deletions ranging in size from a single exon to deletion of the whole gene except for the first two exons.31 To date about 120 different intragenic mutations of ATP7A have been reported: missense (33%), nonsense (16%) and splice-site mutations (16%), and deletions/insertions/duplications (33%) (HGMD).28, 29, 32
About half of the point mutations (deletions/insertions/duplications and nonsense mutations) are truncating mutations, which are shown or predicted to result in a non-functional truncated protein. These truncating mutations are distributed almost equally throughout the gene, and they are predicted to have detrimental effects on the protein function. One interesting aspect of distribution of the mutations is that in the copper-binding domains of ATP7A encoded by exons 2–7, no missense mutations are observed. This suggests that variations in this region are more acceptable and do not necessarily lead to MD. Almost all the disease-causing missense mutations affect residues, which are within the regions conserved among ATPases. Several of the missense mutations leading to amino-acid substitutions have been investigated by functional studies for their effect on protein function (reviewed by de Bie et al35). The mutations may affect protein synthesis, stability of the protein, trafficking and localization, catalytic activity, and post-translational modifications. Missense mutations within the stalk regions or the transmembrane domains may lead to partially functional protein variants containing single-amino-acid substitution. In contrast, missense mutations in the domains important in catalysis (A- and P-domains) are poorly tolerated.32
Effect of the mutation on the phenotype
There is no obvious correlation between the mutations and the clinical course of MD. This is underscored by the presence of inter- and even intra-familial phenotypic variability in MD/OHS patients carrying the same ATP7A mutation.36, 37
In general patients with a milder phenotype (like OHS) have a higher proportion of mutations, which lead to a partially functional protein or result in reduced amounts of an otherwise normal protein.32 Splice-site mutations normally disturb the splicing process and lead to skipping of one or more exons. However, in some cases splice-site mutations do not lead to full disturbance of normal splicing and small amounts of normal transcript (and hence normal protein) can be produced. This has been observed in several OHS patients38, 39, 40 and in patients with a milder phenotype.41 Presence of partially functional, truncated protein variants missing only part of the C-terminus might also result in OHS or a mild Menkes phenotype.31, 42 Interestingly, in a patient with a mild phenotype we have detected deletion of exons 3–4, which resulted in a premature stop codon just at the beginning of exon 5, but translation was reinitiated from a downstream start codon.43 This truncated protein was partially functional with only two copper-binding domains (MBD 5–6), resulting in milder symptoms.
Another mutational mechanism observed in ATP7A is the skipping of exons including the mutations.28 This may also explain why some patients may have milder phenotypes, as these mutations would produce in-frame deletions resulting in a partially functional protein. It is therefore necessary to investigate the effect of ATP7A mutations with functional analyses both at the transcript and protein levels, to be able to predict their effect on the clinical phenotype.
In general gross gene deletions result in the severe classical form of MD, with death in early childhood. However, patients with gross deletions may also have long survival despite severe symptoms. In one patient who died at age 18 years, all the exons of ATP7A except for the first two exons were deleted.31
Animal models
The mottled mouse (mo), which has mutations in atp7a (the mouse orthologue of ATP7A), provides an animal model for MD. Mutations in the mottled locus are common and at least 35 different mutations lead to a wide range of neurological and connective tissue abnormalities. Most of the mottled mutations arise spontaneously and Atp7a mutations have been defined in 12 mottled mutants until now.44, 45, 46
The mottled brindled (Mobr) and mottled macular (Moml) are the closest models to the classical form of MD. They both present with severe neurological impairment and hemizygous males die in the postnatal period (1–3 weeks). Atp7a mutations of both mottled alleles have been identified.47, 48, 49, 50 When Mobr is treated with copper injections within the first week of postnatal period the mice survive and do not develop neurological symptoms.51 Furthermore, transgenic expression of human ATP7A in Mobr could correct the phenotype even though copper defect was not completely corrected.52
The mottled blotchy (Moblo) phenotype resembles OHS by showing predominantly connective tissue manifestations. Moblo has a splice-site mutation (IVS11+3A>C), which affects normal RNA splicing,39 but a normal transcript at a reduced level is also present, as is the case with the mutations of several OHS patients.
Besides the mouse model, two zebrafish mutants, calamity and catastrophe, defective in the orthologue of ATP7A were identified.53, 54 Very recently the phenotypic alterations of two calamity mutant alleles have been corrected by antisense therapy.55
Diagnostic approaches
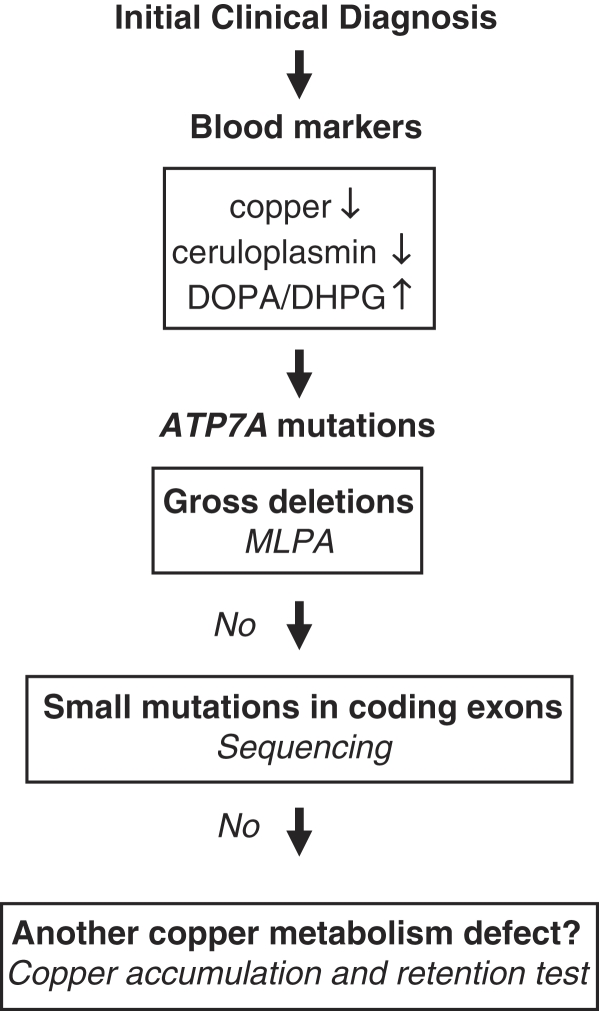
Initial diagnosis of MD is suggested by clinical features (especially typical hair changes) and supported by demonstration of reduced levels of serum copper and ceruloplasmin (Figure 6). However, in the neonatal period these markers should be interpreted with caution, as their levels are low also in healthy newborns. In this period, plasma catecholamine analysis (ratio of DOPA to dihydroxyphenylglycol) indicative of dopamine β-hydroxylase deficiency may be the choice as a rapid diagnostic test.41
Figure 6.
Flow chart for diagnosis. The DOPA/DHPG ratio (ratio of dihydroxyphenylalanine to dihydroxyphenylglycol) is indicative of dopamine β-hydroxylase, which is a copper-dependent enzyme. Gross deletions were previously investigated with the Southern blot method, which is now replaced by MLPA (multliplex ligation-dependent probe amplification) method (LBM, unpublished data).
Other laboratory investigations such as cystourethrography, arteriography, computed tomography, magnetic resonance imaging, and radiography are useful in detecting different clinical features of MD.
Radiographs of patients with classical MD show a number of specific abnormalities that are reminiscent of acquired copper deficiency and scurvy. These changes include generalized osteoporosis, metaphyseal flaring and spurs in the long bones, diaphyseal periosteal reaction and thickening, and Wormian bones in the cranial sutures. Rib fracture due to osteoporosis is a common finding and may lead to misdiagnosis of battered child syndrome. In OHS patients occipital horns are characteristic radiographic findings (Figure 3). These protrusions may be found around 1–2 years of age, but are usually detected only around 5–10 years of age. They continue to grow up to early adulthood.
Light microscopy of hair shows individual hairs that are twisted about their own axes (pili torti), with varying shaft diameters (monilethrix), and fragmentation at regular intervals (trichorrhexis nodosa) (Figure 2).
A definitive biochemical test for MD exists and is based on intracellular accumulation of copper due to impaired efflux. Accumulation is evaluated in cultured cells, mainly fibroblasts, by measuring radioactive copper (64Cu) retention after a 20-h pulse, and impaired efflux is directly determined after a 24-h pulse–chase period. However, these analyses demand expertise and are performed only in a few specialized centers around the world.56
The ultimate diagnostic proof of MD is the demonstration of the molecular defect in ATP7A. However, because of the large size of the gene and the variety of the mutations observed in different families, detection of the genetic defect in a given family may take time.
Carrier identification and prenatal diagnosis
In at-risk families only male fetuses need to be evaluated, and rapid sex determination can be made using Y-chromosome-specific DNA sequences.
Carrier determination by measuring radioactive copper accumulation in cultured fibroblasts is not reliable due to random X-inactivation, and mutation analysis should be performed. In informative families, the intragenic polymorphic markers may also be used for carrier detection.
In at-risk pregnancies where the mutation of the family is unknown, biochemical analysis remains a possibility as identification of the genetic defect may be challenging in limited time. In the first trimester the total copper content in chorionic villi can be measured directly by sensitive and accurate methods like neutron activation analysis and atomic absorption, and in the second trimester copper accumulation is measured in cultured amniotic fluid cells. Although there are potential pitfalls for these analyses, they have been performed routinely at the Kennedy Center in Denmark since 1975.56
Treatment
MD is a progressive disorder leading to death in early childhood in its severe forms, although some patients survive above 5 years of age. Treatment in major cases is mainly symptomatic and clinical reports suggest that care is an important factor in enhancing survival. However, careful medical care, and possibly copper administration, may extend life span up to 13 years or even more. A number of severely affected MD patients with long survival have been reported.
The objective of a specific treatment for MD is to provide extra copper to the tissues and copper-dependent enzymes. Oral administration of copper is ineffective as copper is trapped in the intestines; it should be supplemented parenterally or subcutaneously. Among the available copper compounds, copper histidine has proved to be the most effective.57, 58 Positive outcome of copper–histidine supplementation is dependent on early initiation and presence of at least partially functional ATP7A.
Early, parenteral copper–histidine supplementation may modify disease progression substantially, and the long-term clinical outcome of four early-treated MD cases has been reviewed.58 All these patients clearly exhibited a milder neurological course without seizures, mild-to-moderate ataxia, and near-normal intellectual development. Some problems related to autonomic failure, such as postural hypotension and chronic diarrhea, persisted, and in one patient these symptoms could be corrected by -DOPS. Copper–histidine treatment, however, could not prevent skeletal abnormalities and they have developed some features common to OHS patients. One of these patients is almost 30 years old now (manuscript in preparation).
Early, subcutaneous copper–histidine treatment has also been described recently for 12 younger patients (the oldest patient is 8 years old), with good results.59 However, copper–histidine treatment cannot be accepted as a definitive cure for MD, despite some reported successful outcomes.
The authors declare no conflict of interest.
References
- Tümer Z, Horn N.Menkes diseasein Roach ES, Miller VS (eds): Neurocutaneous Syndromes Cambridge: Cambridge University Press; 2004222–233. [Google Scholar]
- Horn N, Tümer Z.Menkes disease and the occipital horn syndromein Royce PM, Steinmann B (eds): Connecitive Tissue and its Heritable Disorders: Molecular, Genetic, and Medical Aspects New York: John Wiley and Sons Inc.2002. 2nd edn651–685. [Google Scholar]
- Lee J, Penea MMO, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;25:40253–40259. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Speisky H, Gómez M, Burgos-Bravo F, et al. Generation of superoxide radicals by copper-glutathione complexes: redox-consequences associated with their interaction with reduced glutathione. Bioorg Med Chem. 2009;17:1803–1810. doi: 10.1016/j.bmc.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Culotta VC, Yang M, O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem. 2009;284:717–721. doi: 10.1074/jbc.R800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci USA. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camakaris J, Petris MJ, Bailey L, et al. Gene amplification of the Menkes (MNK; ATP7A) P-type ATPase gene of CHO cells is associated with copper resistance and enhanced copper efflux. Hum Mol Genet. 1995;4:2117–2123. doi: 10.1093/hmg/4.11.2117. [DOI] [PubMed] [Google Scholar]
- Monty JF, Llanos RM, Mercer JF, Kramer DR. Copper exposure induces trafficking of the menkes protein in intestinal epithelium of ATP7A transgenic mice. J Nutr. 2005;135:2762–2766. doi: 10.1093/jn/135.12.2762. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- Linder MC, Zerounian NR, Moriya M, Malpe R. Iron and copper homeostasis and intestinal absorption using the Caco2 cell model. Biometals. 2003;16:145–160. doi: 10.1023/a:1020729831696. [DOI] [PubMed] [Google Scholar]
- Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- Meyer LA, Durley AP, Prohaska JR, Harris ZL. Copper transport and metabolism are normal in aceruloplasminemic mice. J Biol Chem. 2001;276:36857–36861. doi: 10.1074/jbc.M105361200. [DOI] [PubMed] [Google Scholar]
- Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood–CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA. Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment. Neuroscience. 2006;139:947–964. doi: 10.1016/j.neuroscience.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Furuyama T, Yamashita S, Mori N. Expression of copper trafficking genes in the mouse brain. Neuroreport. 1998;9:3259–3263. doi: 10.1097/00001756-199810050-00023. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chen YW, Centeno JA, Quezado M, Lem K, Kaler SG. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Mol Genet Metab. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochim Biophys Acta. 1998;1365:37–45. doi: 10.1016/s0005-2728(98)00041-3. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, LeShane ES, Shinde U. Biochemical basis of regulation of human copper-transporting ATPases. Arch Biochem Biophys. 2007;463:134–148. doi: 10.1016/j.abb.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn N, Morton NE. Genetic epidemiology of Menkes disease. Genet Epidemiol. 1986;3:225–230. doi: 10.1002/gepi.1370030403. [DOI] [PubMed] [Google Scholar]
- Tønnesen T, Kleijer WJ, Horn N. Incidence of Menkes disease. Hum Genet. 1991;86:408–410. doi: 10.1007/BF00201846. [DOI] [PubMed] [Google Scholar]
- Gu YH, Kodama H, Shiga K, Nakata S, Yanagawa Y, Ozawa H. A survey of Japanese patients with Menkes disease from 1990 to 2003: incidence and early signs before typical symptomatic onset, pointing the way to earlier diagnosis. J Inherit Metab Dis. 2005;28:473–478. doi: 10.1007/s10545-005-0473-3. [DOI] [PubMed] [Google Scholar]
- Danks DM.Disorders of copper transportin Scriver JR, Beaudet AL, Sly WS, Valle D (eds): The Metabolic Basis of Inherited Disease New York: McGraw-Hill; 1995, 2nd edn, pp2211–2235. [Google Scholar]
- Tümer Z, Vural B, Tønnesen T, Chelly J, Monaco AP, Horn N. Characterization of the exon structure of the Menkes disease gene using Vectorette PCR. Genomics. 1995;26:437–442. doi: 10.1016/0888-7543(95)80160-n. [DOI] [PubMed] [Google Scholar]
- Das S, Levinson B, Whitney S, Vulpe C, Packman S, Gitschier J. Diverse mutations in patients with Menkes disease often lead to exon skipping. Am J Hum Genet. 1994;55:883–889. [PMC free article] [PubMed] [Google Scholar]
- Tümer Z, Møller LB, Horn N. Mutation spectrum of ATP7A, the gene defective in Menkes disease. Adv Exp Med Biol. 1999;448:83–95. doi: 10.1007/978-1-4615-4859-1_7. [DOI] [PubMed] [Google Scholar]
- Gu YH, Kodama H, Murata Y, et al. ATP7A gene mutations in 16 patients with Menkes disease and a patient with occipital horn syndrome. Am J Med Genet. 2001;99:217–222. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1167>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tümer Z, Møller LB, Horn N. Screening of 383 unrelated patients with Menkes disease and finding of 57 gross deletions in ATP7A. Hum Mutat. 2003;22:457–463. doi: 10.1002/humu.10287. [DOI] [PubMed] [Google Scholar]
- Møller LB, Bukrinsky JT, Mølgaard A, et al. Identification and analysis of 21 novel disease-causing amino acid substitutions in the conserved part of ATP7A. Hum Mutat. 2005;26:84–93. doi: 10.1002/humu.20190. [DOI] [PubMed] [Google Scholar]
- Tümer Z, Tommerup N, Tønnesen T, Kreuder J, Craig IW, Horn N. Mapping of the Menkes locus to Xq13.3 distal to the X-inactivation center by an intrachromosomal insertion of the segment Xq13.3–q21.2. Hum Genet. 1992;88:668–672. doi: 10.1007/BF02265295. [DOI] [PubMed] [Google Scholar]
- Sirleto P, Surace C, Santos H, et al. Lyonization effects of the t(X;16) translocation on the phenotypic expression in a rare female with Menkes disease. Pediatr Res. 2009;65:347–351. doi: 10.1203/PDR.0b013e3181973b4e. [DOI] [PubMed] [Google Scholar]
- de Bie P, Muller P, Wijmenga C, Klomp LW. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J Med Genet. 2007;44:673–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borm B, Møller LB, Hausser I, et al. Variable clinical expression of an identical mutation in the ATP7A gene for Menkes disease/occipital horn syndrome in three affected males in a single family. J Pediatr. 2004;145:119–121. doi: 10.1016/j.jpeds.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Donsante A, Tang J, Godwin SC, et al. Differences in ATP7A gene expression underlie intrafamilial variability in Menkes disease/occipital horn syndrome. J Med Genet. 2007;44:492–497. doi: 10.1136/jmg.2007.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson B, Gitschier J, Vulpe C, Whitney S, Yang S, Packman S. Are X-linked cutis laxa and Menkes disease allelic. Nat Genet. 1993;3:6. doi: 10.1038/ng0193-6. [DOI] [PubMed] [Google Scholar]
- Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet. 1995;56:570–576. [PMC free article] [PubMed] [Google Scholar]
- Møller LB, Tümer Z, Lund C, et al. Similar splice-site mutations of the ATP7A gene lead to different phenotypes: classical Menkes disease or occipital horn syndrome. Am J Hum Genet. 2000;66:1211–1220. doi: 10.1086/302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG. Menkes disease. Adv Ped. 1994;41:263–304. [PubMed] [Google Scholar]
- Dagenais SL, Adam AN, Innis JW, Glover TW. A novel frameshift mutation in exon 23 of ATP7A (MNK) results in occipital horn syndrome and not in Menkes disease. Am J Hum Genet. 2001;69:420–427. doi: 10.1086/321290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen M, Lund C, Akram Z, Winther JR, Horn N, Møller LB. Evidence that translation reinitiation leads to a partially functional Menkes protein containing two copper-binding sites. Am J Hum Genet. 2006;79:214–229. doi: 10.1086/505407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe P, Reed V, Boyd Y. Intragenic deletions at Atp7a in mouse models for Menkes disease. Genomics. 2001;74:155–162. doi: 10.1006/geno.2001.6529. [DOI] [PubMed] [Google Scholar]
- Mototani Y, Miyoshi I, Okamura T, et al. Phenotypic and genetic characterization of the Atp7a(Mo-Tohm) mottled mouse: a new murine model of Menkes disease. Genomics. 2006;87:191–199. doi: 10.1016/j.ygeno.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kotula-Balak M, Lenartowicz M, Kowal M, Styrna J, Bilińska B. Testicular morphology and expression of aromatase in testes of mice with the mosaic mutation (Atp7a mo-ms) Theriogenology. 2007;67:423–434. doi: 10.1016/j.theriogenology.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Grimes A, Hearn CJ, Lockhart P, Newgreen DF, Mercer JF. Molecular basis of the brindled mouse mutant (Mo(br)): a murine model of Menkes disease. Hum Mol Genet. 1997;6:1037–1042. doi: 10.1093/hmg/6.7.1037. [DOI] [PubMed] [Google Scholar]
- Reed V, Boyd Y. Mutation analysis provides additional proof that mottled is the mouse homologue of Menkes' disease. Hum Mol Genet. 1997;6:417–423. doi: 10.1093/hmg/6.3.417. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Shiraishi N, Nishikimi M. Occurrence of two missense mutations in Cu-ATPase of the macular mouse, a Menkes disease model. Biochem Mol Biol Int. 1997;43:913–918. doi: 10.1080/15216549700204721. [DOI] [PubMed] [Google Scholar]
- Mori M, Nishimura M. A serine-to-proline mutation in the copper-transporting P-type ATPase gene of the macular mouse. Mamm Genome. 1997;8:407–410. doi: 10.1007/s003359900457. [DOI] [PubMed] [Google Scholar]
- Fujii T, Ito M, Tsuda H, Mikawa H. Biochemical study on the critical period for treatment of the mottled brindled mouse. J Neurochem. 1990;55:885–889. doi: 10.1111/j.1471-4159.1990.tb04574.x. [DOI] [PubMed] [Google Scholar]
- Llanos RM, Ke BX, Wright M, et al. Correction of a mouse model of Menkes disease by the human Menkes gene. Biochim Biophys Acta. 2006;1762:485–493. doi: 10.1016/j.bbadis.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Madsen EC, Gitlin JD. Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism. PLoS Genet. 2008;4:e1000261. doi: 10.1371/journal.pgen.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen EC, Morcos PA, Mendelsohn BA, Gitlin JD. In vivo correction of a Menkes disease model using antisense oligonucleotides. Proc Natl Acad Sci USA. 2008;105:3909–3914. doi: 10.1073/pnas.0710865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer Z, Horn N. Menkes disease: recent advances and new aspects. J Med Genet. 1997;34:265–274. doi: 10.1136/jmg.34.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuder J, Otten A, Fuder H, et al. Clinical and biochemical consequences of copper–histidine therapy in Menkes disease. Eur J Pediatr. 1993;152:828–832. doi: 10.1007/BF02073380. [DOI] [PubMed] [Google Scholar]
- Christodoulou J, Danks DM, Sarkar B, et al. Early treatment of Menkes disease with parenteral copper-histidine: long-term follow-up of four treated patients. Am J Med Genet. 1998;76:154–164. [PubMed] [Google Scholar]
- Kaler SG, Holmes CS, Goldstein DS, et al. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]