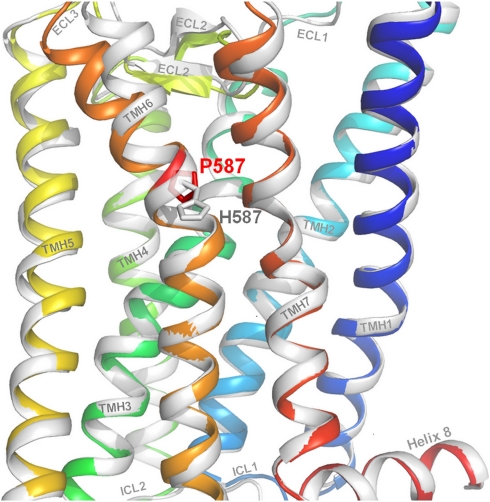
Figure 3.
Molecular homology models of the wild-type and the mutated hFSHR. The backbones of wild-type (coloured rainbow) and Pro-His mutated hFSHR (white) are overlaid. The wild-type amino acid, Proline, is shown at position 587 as well as the mutated histidine side chain. The kink distortion in helix 6 (orange) is caused by the proline and intramolecular side chain interaction between the specifically arranged helical conformations (eg, TMH6 to TMH5 and TMH7). The kink is an important pivot during receptor activation. Substitution of Pro to His is likely to cause structural modifications in TMH6 and consequently also between structural components, eg, the important interfaces between TMH5 and TMH6 or between TMH6 and ECL2. The evoked shift therefore changes stabilizing intramolecular interactions between the helices and leads to a loss of intrinsic signaling capability because of an altered basal conformation.