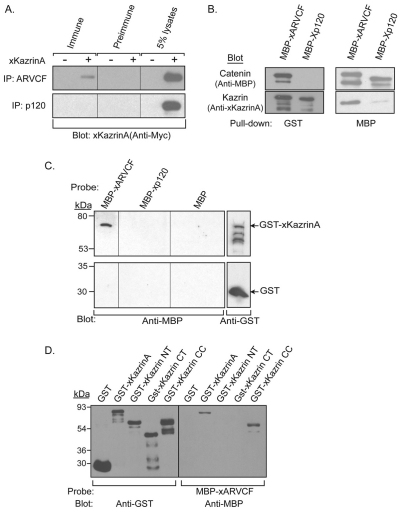
Fig. 2.
Association of full-length xKazrinA with xARVCF. (A) Xenopus embryos expressing HA–xARVCF or HA–Xp120 with (+) or without (−) coexpressed Myc–xKazrinA were harvested at gastrula stage (stage 10.5). Lysates were immunoprecipitated using pre-immune, xARVCF or Xp120 polyclonal antisera, and interactions with xKazrinA detected by immunoblotting using anti-Myc antibody. Total lysates (5%) were also blotted. (B) In vitro co-precipitation of GST–xKazrinA with MBP–xARVCF. GST–xKazrinA was incubated with MBP–xARVCF or MBP–Xp120, precipitated using glutathione–agarose (left panels) or amylose–agarose (right panels) beads and the samples subjected to SDS-PAGE and immunoblotting using anti-MBP (top panels) or anti-Kazrin (bottom panels) antibodies. (C) Direct interaction of purified xARVCF with xKazrinA using blot overlay. Top panel: Duplicate lanes of purified GST–xKazrinA were electrophoresed and immobilized on nitrocellulose then probed using purified MBP–xARVCF, MBP–Xp120 or MBP. Protein complexes were then identified using anti-MBP antibodies (top-left panel). Presence of GST–xKazrinA was detected using anti-GST antibodies (top-right panel). As a negative control, purified GST was immobilized on nitrocellulose and processed in an identical manner (bottom panels). (D) Blot overlay of GST–xKazrinA deletion constructs. Purified GST–xKazrinA, GST–xKazrinA N-terminal (NT), GST–xKazrinA C-terminal (CT), GST–xKazrinA coiled-coil (CC), and GST proteins were electrophoresed and immobilized on nitrocellulose. Total loading was detected using anti-GST antibodies (left panel). The right panel was probed with MBP–xARVCF and the resulting protein complexes detected using anti-MBP antibodies.