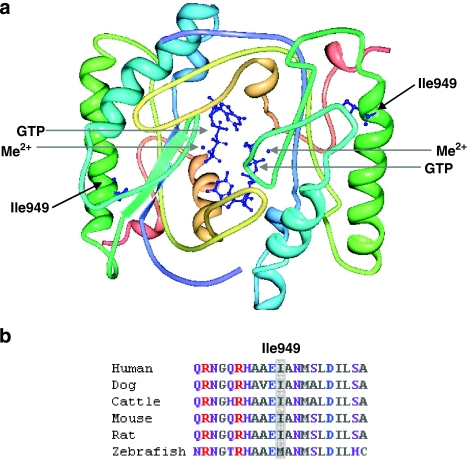
Figure 5.
Structural context of p.Ile949Thr substitution and sequence conservation of Ile949. (a) Theoretical homodimer structure of retGC catalytic domain. The positions of mutated Ile949 residues in alpha helices together with bound Me2+ cofactors and GTP substrates are indicated with arrows, and their atomic structures are shown in blue. The structure was modelled using the Protein Workshop tool (Protein Data Bank, accession ID: 1AWL). (b) ClustalW multiple sequence alignments showing the evolutionary conservation of residue Ile949 (highlighted in grey) among human (Homo sapiens), dog (Canis familiaris), cattle (Bos taurus), mouse (Mus musculus) and rat (Rattus norvegicus), but in zebrafish (Danio rerio) a methionine residue is present at this position. Purple, neutral-polar; red, basic-polar; grey, neutral-nonpolar and blue, acidic-polar amino acids.