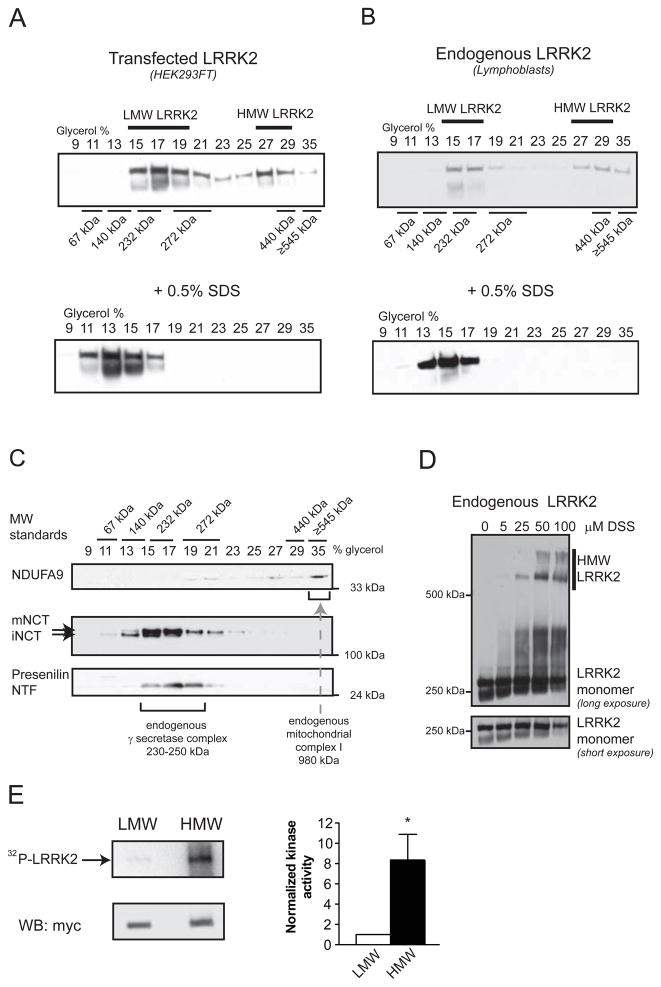
Figure 1. Analysis of HMW and LMW pools of LRRK2 and their associated kinase activity from whole-cell lysates.
(A) Transiently transfected myc-LRRK2 from whole cell lysates is present in two distinct pools, as assessed by glycerol velocity gradients - low molecular weight (LMW) and high molecular weight (HMW). Glycerol gradients were calibrated using commercially available proteins of known molecular weight. Western blots from glycerol gradient fractions show that LMW LRRK2 migrates at ~230 kDa, likely representing a monomer, while HMW LRRK2 is found at approximately double the molecular weight (~440 kDa), consistent with a dimer. Addition of 0.5% SDS prior to glycerol gradients collapses HMW LRRK2.
(B) Endogenous LRRK2 from human lymphoblasts is present in two distinct pools (LMW, HMW), similar to transfected LRRK2. Western blots from glycerol gradient fractions were probed with anti-myc (A) and anti-LRRK2 antibodies (B).
(C) Endogenous γ-secretase complex, a protease of 230–250 kDa, is present in the same glycerol gradient fractions (15–19%) as LMW LRRK2. Cell lysates of HEK293FT cells transfected with myc-LRRK2 were separated using glycerol velocity gradients and analyzed using Western blots for endogenous components of the γ-secretase complex. Immature nicastrin (iNCT) undergoes further glycosylation to generate mature nicastrin (mNCT); only mNCT is part of the fully assembled γ-secretase complex. The N-terminal fragment of presenilin (NTF) is also selectively present in the fully assembled γ-secretase complex. Mitochondrial complex I (980kDa) is found in 35% glycerol, as evidenced by the presence of one its subunits - endogenous NDUFA9.
(D) Chemical crosslinking of live cells leads to the formation of HMW complexes of endogenous LRRK2. Live lymphoblasts expressing endogenous LRRK2 were crosslinked with increasing concentrations of DSS, leading to a dose-dependent formation of SDS-stable HMW LRRK2 complexes.
(E) HMW LRRK2 is more active than LMW. Wild-type myc-LRRK2 from transfected HEK293FT cells was separated into LMW and HMW pools using glycerol gradients, IPed using myc resin and subjected to an autophosphorylation assay of kinase activity. An autoradiograph of an SDS-PAGE gel separating the LRRK2 kinase reaction products shows increased incorporation of radioactive 32P into HMW LRRK2 compared to LMW LRRK2. Similar levels of LRRK2 were present in both reactions, as analyzed by Western blot. Relative kinase activity of HMW LRRK2 is 8.4-fold greater than that of LMW LRRK2 (Mean ± SEM, p = 0.03, n = 6, unpaired t-test). * p < 0.05.