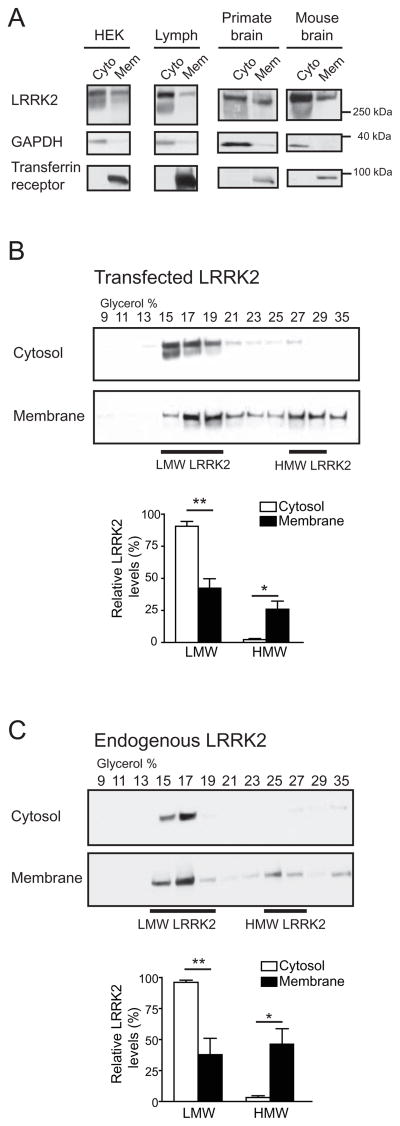
Figure 2. The high molecular weight LRRK2 complex is enriched at the membrane.
(A) A higher proportion of LRRK2 is localized in cytosol than at the membrane. HEK293FT cells (HEK), lymphoblasts (Lymph), primate or mouse brains were homogenized and fractionated into cytosol (Cyto) and membrane (Mem) fractions. Both fractions were volume-normalized and analyzed by SDS-PAGE/Western blot. GAPDH and transferrin receptor were used as controls for proteins in the cytosol and membrane fractions, respectively.
(B) Glycerol gradients of cytosol and membrane extracts from HEK293FT cells transfected with myc-LRRK2 reveal a 20-fold enrichment of HMW LRRK2 at the membrane compared to the cytosol (p = 0.02). A higher proportion of LMW LRRK2 is found in the cytosol than in the membrane compartment (Mean ± SEM, p = 0.004, n = 4, Student’s t-test). * p < 0.05, ** p ≤ 0.01.
(C) Analysis of endogenous LRRK2 from human lymphoblasts reveals an enrichment of its HMW complex at the membrane, similar to that observed in (B) with transfected LRRK2. The levels of HMW LRRK2 are 15-fold higher in membrane than cytosolic fractions (Mean ± SEM, p = 0.01, n = 4, Student’s t-test). * p < 0.05, ** p ≤ 0.01.