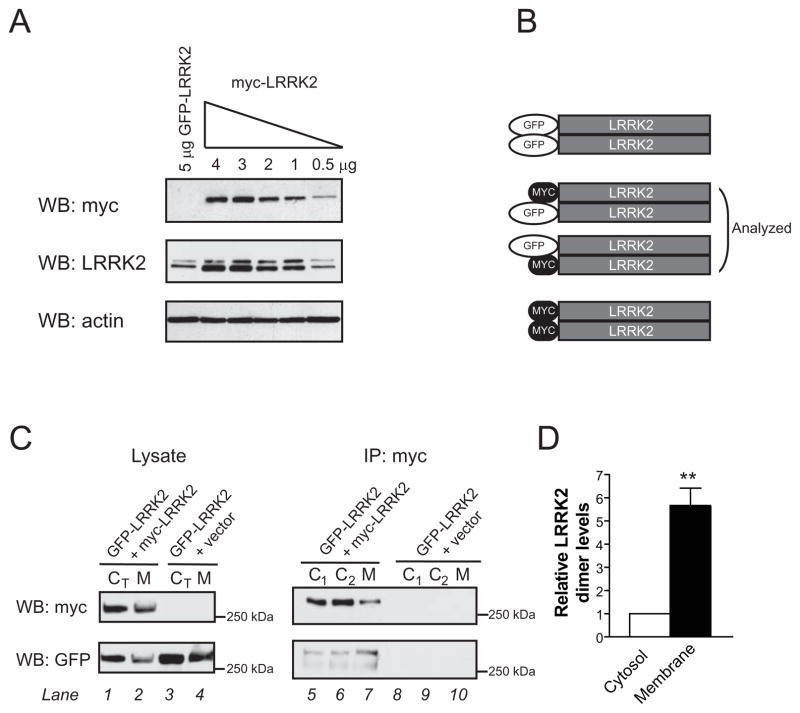
Figure 4. Heterologous co-immunoprecipitation confirms an enrichment of the LRRK2 dimer at the membrane.
(A) To optimize the heterologous co-immunoprecipitation (co-IP) system, the amount of myc-LRRK2 DNA was titrated to match the expression levels of GFP-LRRK2. Transfection with 0.5 μg of myc-LRRK2 led to similar levels of LRRK2 protein as 5 μg of GFP-LRRK2.
(B) A schematic depicting possible LRRK2 dimers after transfection with GFP-LRRK2 and myc-LRRK2 plasmids. Co-IP of heterologously tagged constructs allows quantification of GFP-LRRK2/myc-LRRK2 heterodimer, which at equal levels of both proteins will likely represent 50% of total dimer.
(C) Cytosol and membrane fractions from cells co-expressing GFP-LRRK2 and myc-LRRK2 (lanes 1–2) or GFP-LRRK2 and an empty vector (lanes 3–4) were used for IP using a high affinity myc resin. GFP-LRRK2 is co-IPed only in the presence of myc-LRRK2 (lanes 5–9), confirming specificity of IP. Higher levels of GFP-LRRK2 are pulled down from membrane extracts (lane 7), despite lower levels of myc-LRRK2 in the same IP, suggesting an enrichment of LRRK2 dimer at the membrane. Identical results from two independent samples (C1, C2) using the same total cytosolic fraction (CT) illustrate the low variability of this assay.
(D), Levels of LRRK2 heterodimers are 5.7-fold higher in membrane extracts (Mean ± SEM, p = 0.01, n = 3, unpaired t-test). The relative dimer levels were quantified by measuring the band intensity of co-IPed GFP-LRRK2 divided by the intensity of IPed myc-LRRK2. The value for cytosol was arbitrarily set as 1. ** p ≤ 0.01.