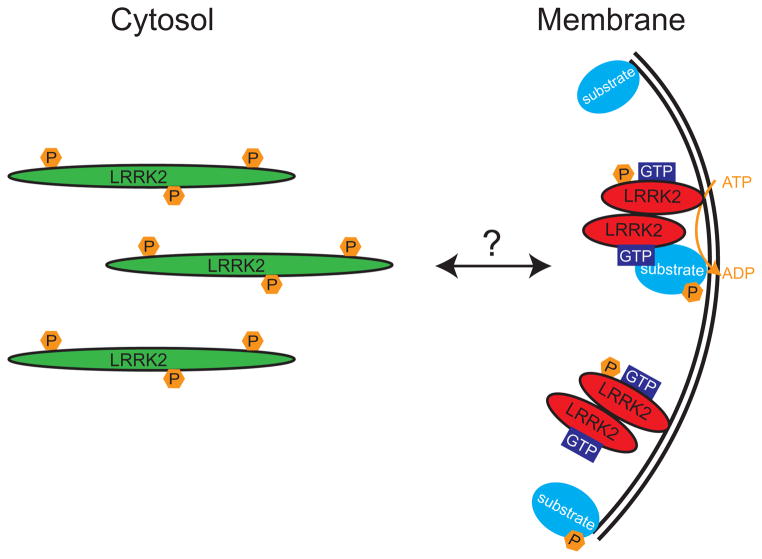
Figure 8. Schematic representation of proposed model of LRRK2 dimer assembly and kinase regulation.
Using both endogenous and exogenous LRRK2, we have observed that membrane-associated LRRK2 is substantially enriched for LRRK2 dimer, whereas cytosolic LRRK2 is present mostly as a monomer. Membrane-associated LRRK2 possesses greater kinase activity, an increased propensity to bind GTP, and is relatively dephosphorylated, compared to cytosolic LRRK2. We propose a model, where LRRK2 exists mostly as a monomer in the cytosol that can translocate to membrane where it dimerizes, becomes more active and subsequently phosphorylates its substrates. The similarities of this model to the established regulation of other kinases (and GTPases) suggest that membrane translocation and dimerization may be reversible and tightly controlled.