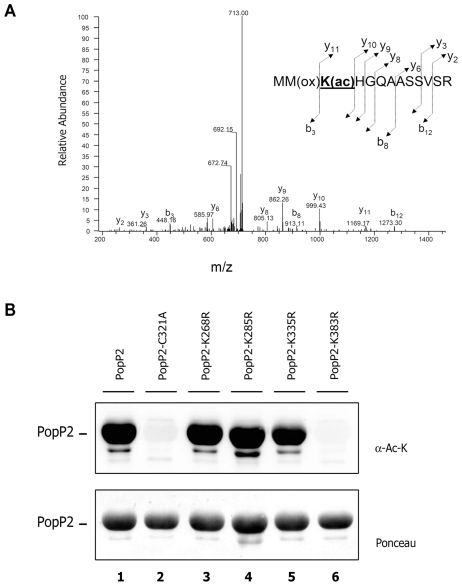
Figure 6. PopP2 is an active acetyl-transferase that autoacetylates on its lysine 383 residue.
A: Mass spectrometric analysis of a PopP2 peptide spanning amino acid residues 381-393. Fragmentation of this peptide led to the identification of an acetylated lysine residue within the sequence MMK(ac)HGQAASSVSR [K(ac) indicates an acetylated lysine], in wild-type PopP2, but not PopP2-C321A. Labels b and y designate the N- and C-terminal fragments, respectively, of the peptide produced by cleavage at the peptide bond in the mass spectrometer. Numbers represent the number of N- or C-terminal residues present in the peptide fragment. B: Recognition of acetylated wild-type PopP2, but not PopP2-C321A or PopP2-K383R, by an anti-acetyl-lysine antibody. GST-PopP2, GST-PopP2-C321A, GST-PopP2-K268R, GST-PopP2-K285R, GST-PopP2-K335R and GST-PopP2-K383R proteins were expressed in E. coli and analysed by immunoblot using an antibody that recognizes acetylated lysines. GST-purified PopP2 recombinant proteins are shown after Ponceau staining (bottom).