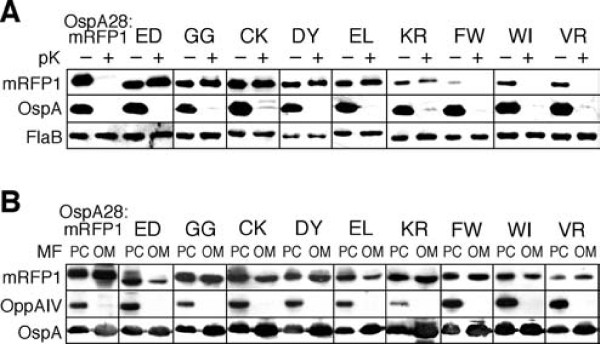
Figure 4.
Phenotypical analysis of select OspA:mRFP1 fusion mutants. Representative Western blots of select mutants are shown (see Additional File 2-Figures S1 and S2 for full data set). Mutant-specific amino acid sequences are listed in single letter code above the blots. OspA28:mRFP1 and OspA20:mRFP1 (ED) were included as controls. (A) Protein expression and protease accessibility. Whole cell lysates of B. burgdorferi expressing mutant OspA:mRFP1 fusions from an identical PflaB promoter (Figure 1) were obtained before (-) or after (+) in situ treatment with proteinase K (pK). A polyclonal antiserum against mRFP1 was used to detect the OspA:mRFP1 fusions. Constitutively expressed periplasmic FlaB was used as a control for loading (to normalize signals within samples) as well as for subsurface localization (negative control). OspA served as a surface control. Untreated (-pK) samples were used to assess protein expression/in vivo stability of OspA:mRFP1 fusions. (B) Distribution of proteins to inner or outer membranes. Protoplasmic cylinder (PC) and outer membrane vesicle (OM) fractions from B. burgdorferi expressing mutant OspA:mRFP1 fusions were probed with a polyclonal antiserum against mRFP1 to detect the OspA:mRFP1 fusions. IM-localized lipoprotein OppAIV was used as a PC-specific control. Surface lipoprotein OspA was used as an outer membrane control. Note that the PC fraction also contains intact cells, i.e. also contains OM proteins.