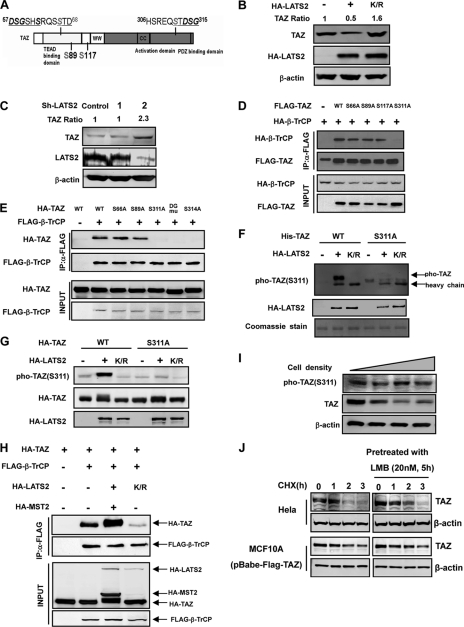
FIGURE 2.
Phosphorylation of Ser-311 is important for TAZ to bind with β-TrCP. A, schematic illustration of TAZ structure showing the two putative phosphodegrons. The sequences surrounding the phosphodegrons are shown. Lats phosphorylation serine residues are indicated in gray. B, ectopic expression of LATS2 decreases the steady state level of TAZ. The indicated plasmids were co-transfected into HEK293 cells. K/R denotes the kinase inactive mutant LATS2. Relative TAZ levels were quantified by the ratio of TAZ to actin. C, LATS2 knocking down increases endogenous TAZ protein level. LATS2 or unrelated control shRNA was transfected into 293 cells, and endogenous TAZ protein level was examined. LATS2 knockdown efficiency was determined by WB. Relative TAZ levels were quantified by the ratio of TAZ to actin. D, mutation of the LATS phosphorylation site S311A disrupts the interaction between TAZ and β-TrCP. β-TrCP was co-transfected with TAZ WT or different mutants. FLAG-TAZ was immunoprecipitated and the associated HA-β-TrCP was detected by HA WB. The TAZ S311A mutant showed weak interaction with β-TrCP. E, mutations of the C-terminal phosphodegron disrupt the binding between TAZ and β-TrCP. Experiments were similar to those in panel D. Different TAZ mutants used in the transfection are indicated. F, Ser-311 is phosphorylated by LATS in vitro. His-TAZ and S311A mutant were expressed in E. coli and purified. HA-LATS2 was immunoprecipitated from transfected 293T cells and used to phosphorylate the purified His-TAZ in vitro. Phosphorylation of TAZ was detected by pTAZ (Ser-311) antibody. His-TAZ was shown by Coomassie Blue staining. G, expression of LATS enhances TAZ Ser-311 phosphorylation in transfected cells. TAZ WT or S311A mutant was co-transfected with WT or kinase inactive (K/R) mutant of LATS2. Phosphorylation of the co-transfected TAZ was detected by the pTAZ(311) antibody. H, MST/LATS co-transfection increases the interaction between TAZ and β-TrCP. Indicated plasmids were co-transfected into HEK293 cells. FLAG-β-TrCP was immunoprecipitated with FLAG antibody and the co-precipitated HA-TAZ was detected by HA WB. I, TAZ protein level decreases with cell density. MCF10A cells were cultured from 30% density to confluent. TAZ protein levels were determined by WB. J, leptomycin B treatment results in a detectable increase of TAZ stability. Both Hela and Flag-TAZ expressed MCF10A cells were pretreated with or without 20 nm leptomycin B for 5 h, followed by treatment with CHX (20 μg/ml) for indicated times. Endogenous or Flag-TAZ protein levels were determined.