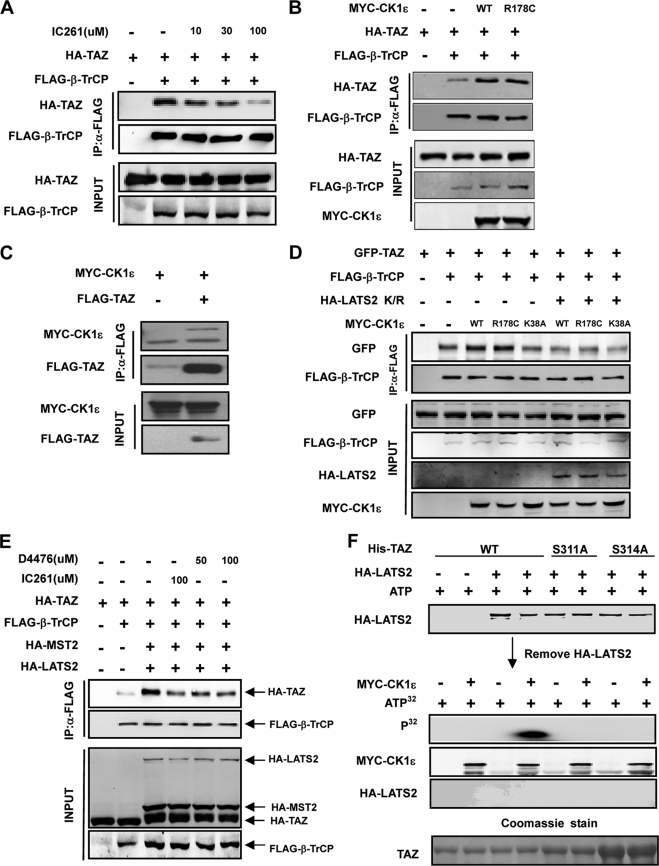
FIGURE 3.
Casein kinase I cooperates with LATS to regulate the interaction between TAZ and β-TrCP. A, IC261 treatment disrupts the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected and cells were treated with or without IC261, which is a CKIϵ/δ specific inhibitor. The interaction between TAZ and β-TrCP was analyzed by co-IP followed by WB. B, CK1ϵ promotes the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected. The association between TAZ and β-TrCP was examined by co-IP. R178C is an active mutant of CKIϵ. C, association between CK1ϵ and TAZ. CKIϵ was co-transfected with or without TAZ. co-IP was performed to examine the interaction between TAZ and CKIϵ. D, kinase inactive LATS2 mutant blocks the interaction between TAZ and β-TrCP induced by CK1ϵ. The indicated plasmids were co-transfected into HEK293 cells. Interaction between TAZ and β-TrCP was analyzed by co-IP. E, CK1ϵ activity is required for Mst2/LATS2 to stimulate the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected, and cells were treated with or without IC261 or D4476. The interaction between TAZ and β-TrCP was analyzed by co-IP. F, phosphorylation of TAZ S314 by CKIϵ requires LATS2 priming phosphorylation. His-TAZ WT or mutants were expressed and purified from E. coli. The purified TAZ was incubated with HA-LATS2 immunoprecipitated from transfected HEK293T cells, in the presence of cold ATP. HA-LATS2 was removed from the kinase reaction. The prime phosphorylated His-TAZ was then incubated with Myc-CK1ϵ immunoprecipitated from transfected HEK293T cells, in the presence of radioactive ATP. Phosphorylation of TAZ by CKIϵ was detected by incorporation of 32P. His-TAZ input was shown by Coomassie Blue staining (bottom panel).